The Caenorhabditis elegans epidermis as a model skin. I: development, patterning, and growth
- PMID: 23539299
- PMCID: PMC3607643
- DOI: 10.1002/wdev.79
The Caenorhabditis elegans epidermis as a model skin. I: development, patterning, and growth
Abstract
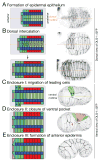
The skin of the nematode Caenorhabditis elegans is composed of a simple epidermal epithelium and overlying cuticle. The skin encloses the animal and plays central roles in body morphology and physiology; its simplicity and accessibility make it a tractable genetic model for several aspects of skin biology. Epidermal precursors are specified by a hierarchy of transcriptional regulators. Epidermal cells form on the dorsal surface of the embryo and differentiate to form the epidermal primordium, which then spreads out in a process of epiboly to enclose internal tissues. Subsequent elongation of the embryo into a vermiform larva is driven by cell shape changes and cell fusions in the epidermis. Most epidermal cells fuse in mid-embryogenesis to form a small number of multinucleate syncytia. During mid-embryogenesis the epidermis also becomes intimately associated with underlying muscles, performing a tendon-like role in transmitting muscle force. Post-embryonic development of the epidermis involves growth by addition of new cells to the syncytia from stem cell-like epidermal seam cells and by an increase in cell size driven by endoreplication of the chromosomes in epidermal nuclei.
Figures




References
-
- Thompson DP, Geary TG. Excretion/Secretion, Ionic and Osmotic Regulation. In: Lee DL, editor. The Biology of Nematodes. Boca Raton, FL: CRC Press; 2002. pp. 291–320.
-
- Payre F. Genetic control of epidermis differentiation in Drosophila. Int J Dev Biol. 2004;48:207–215. - PubMed
-
- Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio) The International journal of developmental biology. 2004;48:217–231. - PubMed
-
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Ann Rev Cell Dev Biol. 2007;23:93–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

