Navigating the B(12) road: assimilation, delivery, and disorders of cobalamin
- PMID: 23539619
- PMCID: PMC3650358
- DOI: 10.1074/jbc.R113.458810
Navigating the B(12) road: assimilation, delivery, and disorders of cobalamin
Abstract
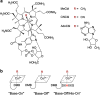
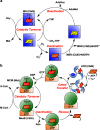
The reactivity of the cobalt-carbon bond in cobalamins is the key to their chemical versatility, supporting both methyl transfer and isomerization reactions. During evolution of higher eukaryotes that utilize vitamin B12, the high reactivity of the cofactor coupled with its low abundance pressured development of an efficient system for uptake, assimilation, and delivery of the cofactor to client B12-dependent enzymes. Although most proteins suspected to be involved in B12 trafficking were discovered by 2009, the recent identification of a new protein reveals that the quest for elucidating the intracellular B12 highway is still far from complete. Herein, we review the biochemistry of cobalamin trafficking.
Keywords: Adenosylcobalamin; Cofactors; Metabolic Diseases; Metal Homeostasis; Trafficking; Vitamins.
Figures


References
-
- Waldron K. J., Rutherford J. C., Ford D., Robinson N. J. (2009) Metalloproteins and metal sensing. Nature 460, 823–830 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

