Podocytes are nonhematopoietic professional antigen-presenting cells
- PMID: 23539760
- PMCID: PMC3665387
- DOI: 10.1681/ASN.2012020133
Podocytes are nonhematopoietic professional antigen-presenting cells
Abstract
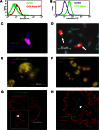
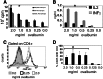
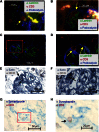
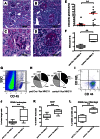
Podocytes are essential to the structure and function of the glomerular filtration barrier; however, they also exhibit increased expression of MHC class II molecules under inflammatory conditions, and they remove Ig and immune complexes from the glomerular basement membrane (GBM). This finding suggests that podocytes may act as antigen-presenting cells, taking up and processing antigens to initiate specific T cell responses, similar to professional hematopoietic cells such as dendritic cells or macrophages. Here, MHC-antigen complexes expressed exclusively on podocytes of transgenic mice were sufficient to activate CD8+ T cells in vivo. In addition, deleting MHC class II exclusively on podocytes prevented the induction of experimental anti-GBM nephritis. Podocytes ingested soluble and particulate antigens, activated CD4+ T cells, and crosspresented exogenous antigen on MHC class I molecules to CD8+ T cells. In conclusion, podocytes participate in the antigen-specific activation of adaptive immune responses, providing a potential target for immunotherapies of inflammatory kidney diseases and transplant rejection.
Figures



References
-
- Goldwich A, Hahn SS, Schreiber S, Meier S, Kämpgen E, Wagner R, Lutz MB, Schubert U: Targeting HIV-1 Gag into the defective ribosomal product pathway enhances MHC class I antigen presentation and CD8+ T cell activation. J Immunol 180: 372–382, 2008 - PubMed
-
- Burgdorf S, Kautz A, Böhnert V, Knolle PA, Kurts C: Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316: 612–616, 2007 - PubMed
-
- Burgdorf S, Schölz C, Kautz A, Tampé R, Kurts C: Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol 9: 558–566, 2008 - PubMed
-
- Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

