Cystathionine gamma-lyase expression is regulated by exogenous hydrogen peroxide in the mammalian cells
- PMID: 23539901
- PMCID: PMC6043835
- DOI: 10.3727/105221613x13571653093286
Cystathionine gamma-lyase expression is regulated by exogenous hydrogen peroxide in the mammalian cells
Abstract
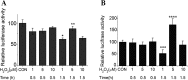
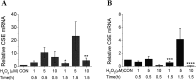
Hydrogen sulfide (H2S), as an endogenous signaling molecule in mammals, shows a variety of biological effects. Cystathionine gamma-lyase (CSE)/H2S pathway has been implicated in scavenging reactive oxygen species (ROS) in the mammalian cells. Therefore, we first investigated the regulatory effects of exogenously applied hydrogen peroxide (H2O2) on CSE expression in the mammalian cells. African green monkey kidney fibroblastlike cells (COS-7 cells) or human embryonic kidney 293 cells (HEK 293 cells) were transfected with CSE promoter-luciferase reporter constructs and treated with H2O2 of 1, 5, and 10 microM for 0.5 and 1.5 h at 37 degrees C. The transfected cells were assayed for firefly luciferase activities normalized by Renilla luciferase activity. Human lung adenocarcinoma cells (A549 cells) or human liver cancer cells (SMMC-7721 cells) were treated with H2O2 of 1, 5, and 10 microM for 0.5 and 1.5 h at 37 degrees C, and were then harvested and analyzed by Western blotting and quantitative RT-PCR. Our results showed that the treatment of a medium concentration (5 microM) of H2O2 at a longer time (1.5 h) upregulated CSE expression in the mammalian cells at the levels of the promoter, message RNA, and protein. Collectively, exogenously applied H2O2 can not only markedly affect CSE mRNA and protein expression, but also can affect the CSE promoter activity in the mammalian cells. Our observations indicate that that exogenous H2O2 can upregulate the expression of the CSE gene in the mammalian cells, which will provide the possibility of the scavenging effect of the CSE gene indirectly on ROS in the mammalian cells. However, the regulatory mechanism involved in the effects of exogenously applied H2O2 on CSE expression in the mammalian cells need be further studied.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures

Similar articles
-
The effect of certain conditions in the regulation of cystathionine γ-lyase by exogenous hydrogen sulfide in mammalian cells.Biochem Genet. 2013 Aug;51(7-8):503-13. doi: 10.1007/s10528-013-9581-1. Epub 2013 Mar 21. Biochem Genet. 2013. PMID: 23515848
-
The binding site for the transcription factor, NF-κB, on the cystathionine γ-lyase promoter is critical for LPS-induced cystathionine γ-lyase expression.Int J Mol Med. 2014 Aug;34(2):639-45. doi: 10.3892/ijmm.2014.1788. Epub 2014 May 27. Int J Mol Med. 2014. PMID: 24866963
-
Regulation of cystathionine γ-lyase in mammalian cells by hypoxia.Biochem Genet. 2014 Feb;52(1-2):29-37. doi: 10.1007/s10528-013-9624-7. Epub 2013 Jul 13. Biochem Genet. 2014. PMID: 23852134
-
Endogenous and exogenous hydrogen sulfide facilitates T-type calcium channel currents in Cav3.2-expressing HEK293 cells.Biochem Biophys Res Commun. 2014 Feb 28;445(1):225-9. doi: 10.1016/j.bbrc.2014.01.185. Epub 2014 Feb 6. Biochem Biophys Res Commun. 2014. PMID: 24508802
-
Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-β- synthase (CBS) and cystathionine-γ-lyase (CSE).Inflamm Allergy Drug Targets. 2011 Apr;10(2):85-91. doi: 10.2174/187152811794776286. Inflamm Allergy Drug Targets. 2011. PMID: 21275900 Review.
Cited by
-
Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes.Sci Rep. 2017 Mar 2;7:42871. doi: 10.1038/srep42871. Sci Rep. 2017. PMID: 28251989 Free PMC article.
-
Role of hydrogen sulfide in brain synaptic remodeling.Methods Enzymol. 2015;555:207-29. doi: 10.1016/bs.mie.2014.11.025. Epub 2015 Jan 13. Methods Enzymol. 2015. PMID: 25747482 Free PMC article. Review.
-
Transcriptional Regulation of Cystathionine-γ-Lyase in Endothelial Cells by NADPH Oxidase 4-Dependent Signaling.J Biol Chem. 2016 Jan 22;291(4):1774-1788. doi: 10.1074/jbc.M115.685578. Epub 2015 Nov 30. J Biol Chem. 2016. PMID: 26620565 Free PMC article.
-
Diurnal Fluctuations in Plasma Hydrogen Sulfide of the Mice.Front Pharmacol. 2017 Oct 6;8:682. doi: 10.3389/fphar.2017.00682. eCollection 2017. Front Pharmacol. 2017. PMID: 29056911 Free PMC article.
-
Redox regulation of gasotransmission in the vascular system: A focus on angiogenesis.Free Radic Biol Med. 2017 Jul;108:500-516. doi: 10.1016/j.freeradbiomed.2017.04.025. Epub 2017 Apr 19. Free Radic Biol Med. 2017. PMID: 28433660 Free PMC article. Review.
References
-
- Bhatia M.; Wong F. L.; Fu D.; Lau H. Y.; Moochhala S. M.; Moore P. K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 19:623–625; 2005. - PubMed
-
- Chang L.; Geng B.; Yu F.; Zhao J.; Jiang H.; Du J.; Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34:573–585; 2008. - PubMed
-
- Choudhary S.; Rosenblatt K. P.; Fang L.; Tian B.; Wu Z. H.; Brasier A. R. High throughput short interfering RNA (siRNA) screening of the human kinome identifies novel kinases controlling the canonical nuclear factor-kappaB (NF-kappaB) activation pathway. J. Biol. Chem. 286:37187–37195; 2011. - PMC - PubMed
-
- Denu J. M.; Tanner K. G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 37:5633–5642; 1998. - PubMed
-
- Dominy J. E.; Stipanuk M. H. New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr. Rev. 62:348–353; 2004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
