Emergence of glass-like behavior in Markov state models of protein folding dynamics
- PMID: 23540906
- PMCID: PMC3677858
- DOI: 10.1021/ja4002663
Emergence of glass-like behavior in Markov state models of protein folding dynamics
Abstract
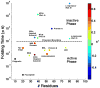
The extent to which glass-like kinetics govern dynamics in protein folding has been heavily debated. Here, we address the subject with an application of space-time perturbation theory to the dynamics of protein folding Markov state models. Borrowing techniques from the s-ensemble method, we argue that distinct active and inactive phases exist for protein folding dynamics, and that kinetics for specific systems can fall into either dynamical regime. We do not, however, observe a true glass transition in any system studied. We go on to discuss how these inactive and active phases might relate to general protein folding properties.
Figures


References
-
- Dobson CM. Sem In Cell and Dev Bio. 2004;15(1):3–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

