The benefits of using genetic information to design prevention trials
- PMID: 23541341
- PMCID: PMC3617383
- DOI: 10.1016/j.ajhg.2013.03.003
The benefits of using genetic information to design prevention trials
Abstract
Clinical trials for preventative therapies are complex and costly endeavors focused on individuals likely to develop disease in a short time frame, randomizing them to treatment groups, and following them over time. In such trials, statistical power is governed by the rate of disease events in each group and cost is determined by randomization, treatment, and follow-up. Strategies that increase the rate of disease events by enrolling individuals with high risk of disease can significantly reduce study size, duration, and cost. Comprehensive study of common, complex diseases has resulted in a growing list of robustly associated genetic markers. Here, we evaluate the utility--in terms of trial size, duration, and cost--of enriching prevention trial samples by combining clinical information with genetic risk scores to identify individuals at greater risk of disease. We also describe a framework for utilizing genetic risk scores in these trials and evaluating the associated cost and time savings. With type 1 diabetes (T1D), type 2 diabetes (T2D), myocardial infarction (MI), and advanced age-related macular degeneration (AMD) as examples, we illustrate the potential and limitations of using genetic data for prevention trial design. We illustrate settings where incorporating genetic information could reduce trial cost or duration considerably, as well as settings where potential savings are negligible. Results are strongly dependent on the genetic architecture of the disease, but we also show that these benefits should increase as the list of robustly associated markers for each disease grows and as large samples of genotyped individuals become available.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
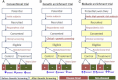
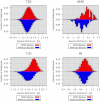
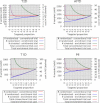
References
-
- Dickson M., Gagnon J.P. Key factors in the rising cost of new drug discovery and development. Nat. Rev. Drug Discov. 2004;3:417–429. - PubMed
-
- Cummings J.L., Doody R., Clark C. Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology. 2007;69:1622–1634. - PubMed
-
- Florez J.C., Jablonski K.A., Bayley N., Pollin T.I., de Bakker P.I., Shuldiner A.R., Knowler W.C., Nathan D.M., Altshuler D., Diabetes Prevention Program Research Group TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 2006;355:241–250. - PMC - PubMed
-
- Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr., Kastelein J.J., Koenig W., Libby P., Lorenzatti A.J., MacFadyen J.G., JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008;359:2195–2207. - PubMed
-
- Simon R. The use of genomics in clinical trial design. Clin. Cancer Res. 2008;14:5984–5993. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

