Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis
- PMID: 23541731
- PMCID: PMC3718648
- DOI: 10.1016/j.cub.2013.02.047
Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis
Abstract
Background: Motile cells exposed to an external direct current electric field will reorient and migrate along the direction of the electric potential in a process known as galvanotaxis. The underlying physical mechanism that allows a cell to sense an electric field is unknown, although several plausible hypotheses have been proposed. In this work we evaluate the validity of each of these mechanisms.
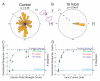
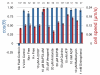
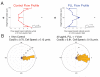
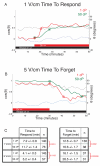
Results: We find that the directional motile response of fish epidermal cells to the cathode in an electric field does not require extracellular sodium or potassium, is insensitive to membrane potential, and is also insensitive to perturbation of calcium, sodium, hydrogen, or chloride ion transport across the plasma membrane. Cells migrate in the direction of applied forces from laminar fluid flow, but reversal of electro-osmotic flow did not affect the galvanotactic response. Galvanotaxis fails when extracellular pH is below 6, suggesting that the effective charge of membrane components might be a crucial factor. Slowing the migration of membrane components with an increase in aqueous viscosity slows the kinetics of the galvanotactic response. In addition, inhibition of PI3K reverses the cell's response to the anode, suggesting the existence of multiple signaling pathways downstream of the galvanotactic signal.
Conclusions: Our results are most consistent with the hypothesis that electrophoretic redistribution of membrane components of the motile cell is the primary physical mechanism for motile cells to sense an electric field. This chemical polarization of the cellular membrane is then transduced by intracellular signaling pathways canonical to chemotaxis to dictate the cell's direction of travel.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Verworn M. Die polare Erregung der Protisten durch den galvanischen strom. (“The polar excitation of protists by galvanic current”) Pflugers Arch Ges Physiol. 1889;45:1–36.
-
- Hotary KB, Robinson KR. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166(2):789–800. doi:10.1006/dbio.1994.1357. - PubMed
-
- McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122(Pt 23):4267–76. doi:10.1242/jcs.023564. - PubMed
-
- Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20(6):674–82. doi:10.1016/j.semcdb.2008.12.009. - PubMed
-
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85(3):943–78. doi:10.1152/physrev.00020.2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

