Redox regulation of SIRT1 in inflammation and cellular senescence
- PMID: 23542362
- PMCID: PMC3762912
- DOI: 10.1016/j.freeradbiomed.2013.03.015
Redox regulation of SIRT1 in inflammation and cellular senescence
Abstract
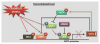
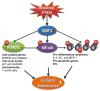
Sirtuin 1 (SIRT1) regulates inflammation, aging (life span and health span), calorie restriction/energetics, mitochondrial biogenesis, stress resistance, cellular senescence, endothelial functions, apoptosis/autophagy, and circadian rhythms through deacetylation of transcription factors and histones. SIRT1 level and activity are decreased in chronic inflammatory conditions and aging, in which oxidative stress occurs. SIRT1 is regulated by a NAD(+)-dependent DNA repair enzyme, poly(ADP-ribose) polymerase-1 (PARP1), and subsequent NAD(+) depletion by oxidative stress may have consequent effects on inflammatory and stress responses as well as cellular senescence. SIRT1 has been shown to undergo covalent oxidative modifications by cigarette smoke-derived oxidants/aldehydes, leading to posttranslational modifications, inactivation, and protein degradation. Furthermore, oxidant/carbonyl stress-mediated reduction of SIRT1 leads to the loss of its control on acetylation of target proteins including p53, RelA/p65, and FOXO3, thereby enhancing the inflammatory, prosenescent, and apoptotic responses, as well as endothelial dysfunction. In this review, the mechanisms of cigarette smoke/oxidant-mediated redox posttranslational modifications of SIRT1 and its roles in PARP1 and NF-κB activation, and FOXO3 and eNOS regulation, as well as chromatin remodeling/histone modifications during inflammaging, are discussed. Furthermore, we have also discussed various novel ways to activate SIRT1 either directly or indirectly, which may have therapeutic potential in attenuating inflammation and premature senescence involved in chronic lung diseases.
Keywords: COPD; FOXO3; Free radicals; GSH; Inflammation; NF-κB; Oxidants; Redox signaling; SIRT1; Senescence; Tobacco smoke.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






References
-
- Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. - PubMed
-
- McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108:652–659. - PubMed
-
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–36. - PubMed
-
- Droge W. The plasma redox state and ageing. Ageing Res Rev. 2002;1:257–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

