A pathogenic picornavirus acquires an envelope by hijacking cellular membranes
- PMID: 23542590
- PMCID: PMC3631468
- DOI: 10.1038/nature12029
A pathogenic picornavirus acquires an envelope by hijacking cellular membranes
Abstract
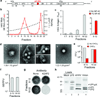
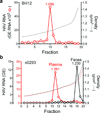
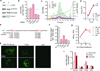
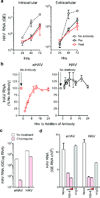
Animal viruses are broadly categorized structurally by the presence or absence of an envelope composed of a lipid-bilayer membrane, attributes that profoundly affect stability, transmission and immune recognition. Among those lacking an envelope, the Picornaviridae are a large and diverse family of positive-strand RNA viruses that includes hepatitis A virus (HAV), an ancient human pathogen that remains a common cause of enterically transmitted hepatitis. HAV infects in a stealth-like manner and replicates efficiently in the liver. Virus-specific antibodies appear only after 3-4 weeks of infection, and typically herald its resolution. Although unexplained mechanistically, both anti-HAV antibody and inactivated whole-virus vaccines prevent disease when administered as late as 2 weeks after exposure, when virus replication is well established in the liver. Here we show that HAV released from cells is cloaked in host-derived membranes, thereby protecting the virion from antibody-mediated neutralization. These enveloped viruses ('eHAV') resemble exosomes, small vesicles that are increasingly recognized to be important in intercellular communications. They are fully infectious, sensitive to extraction with chloroform, and circulate in the blood of infected humans. Their biogenesis is dependent on host proteins associated with endosomal-sorting complexes required for transport (ESCRT), namely VPS4B and ALIX. Whereas the hijacking of membranes by HAV facilitates escape from neutralizing antibodies and probably promotes virus spread within the liver, anti-capsid antibodies restrict replication after infection with eHAV, suggesting a possible explanation for prophylaxis after exposure. Membrane hijacking by HAV blurs the classic distinction between 'enveloped' and 'non-enveloped' viruses and has broad implications for mechanisms of viral egress from infected cells as well as host immune responses.
Figures
Comment in
-
Viral pathogenesis: Cloak and dagger.Nat Rev Microbiol. 2013 Jun;11(6):360. doi: 10.1038/nrmicro3026. Epub 2013 Apr 16. Nat Rev Microbiol. 2013. PMID: 23588217 No abstract available.
-
Hepatitis virus hijacks shuttle: exosome-like vesicles provide protection against neutralizing antibodies.Hepatology. 2014 Jun;59(6):2416-8. doi: 10.1002/hep.26943. Epub 2014 Apr 14. Hepatology. 2014. PMID: 24273053 No abstract available.
References
-
- Harrison SC. In: Fields Virology. Knipe DM, et al., editors. Lippincott Williams & Wilkins; 2007. pp. 59–98. Ch. 3.
-
- Feng Z, Lemon SM. In: The Picornaviruses. Ehrenfeld E, Domingo E, Roos RP, editors. Washington, DC: ASM Press; 2010. pp. 383–396. Ch. 25.
-
- Lemon SM. Type A viral hepatitis: new developments in an old disease. New Engl. J. Med. 1985;313:1059–1067. - PubMed
-
- Martin A, Lemon SM. In: Hepatitis Viruses. Ou J, editor. Kluwer Academic Publishers; 2002. pp. 23–50.
Additional References
-
- Taylor KL, et al. Attenuation phenotype of a cell culture-adapted variant of hepatitis A virus (HM175/p16) in susceptible new world owl monkeys. J Infect Dis. 1993;168:592–601. - PubMed
-
- Lemon SM, et al. Transmission of hepatitis A virus among recently captured Panamanian owl monkeys. J. Med. Virol. 1982;10:25–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

