Biochemical diversity of carboxyl esterases and lipases from Lake Arreo (Spain): a metagenomic approach
- PMID: 23542620
- PMCID: PMC3675924
- DOI: 10.1128/AEM.00240-13
Biochemical diversity of carboxyl esterases and lipases from Lake Arreo (Spain): a metagenomic approach
Abstract
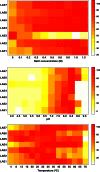
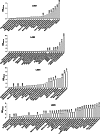
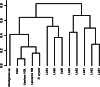
The esterases and lipases from the α/β hydrolase superfamily exhibit an enormous sequence diversity, fold plasticity, and activities. Here, we present the comprehensive sequence and biochemical analyses of seven distinct esterases and lipases from the metagenome of Lake Arreo, an evaporite karstic lake in Spain (42°46'N, 2°59'W; altitude, 655 m). Together with oligonucleotide usage patterns and BLASTP analysis, our study of esterases/lipases mined from Lake Arreo suggests that its sediment contains moderately halophilic and cold-adapted proteobacteria containing DNA fragments of distantly related plasmids or chromosomal genomic islands of plasmid and phage origins. This metagenome encodes esterases/lipases with broad substrate profiles (tested over a set of 101 structurally diverse esters) and habitat-specific characteristics, as they exhibit maximal activity at alkaline pH (8.0 to 8.5) and temperature of 16 to 40°C, and they are stimulated (1.5 to 2.2 times) by chloride ions (0.1 to 1.2 M), reflecting an adaptation to environmental conditions. Our work provides further insights into the potential significance of the Lake Arreo esterases/lipases for biotechnology processes (i.e., production of enantiomers and sugar esters), because these enzymes are salt tolerant and are active at low temperatures and against a broad range of substrates. As an example, the ability of a single protein to hydrolyze triacylglycerols, (non)halogenated alkyl and aryl esters, cinnamoyl and carbohydrate esters, lactones, and chiral epoxides to a similar extent was demonstrated.
Figures


References
-
- Casas-Godoy L, Duquesne S, Bordes F, Sandoval G, Marty A. 2012. Lipases: an overview. Methods Mol. Biol. 861:3–30 - PubMed
-
- Fan X, Niehus X, Sandoval G. 2012. Lipases as biocatalyst for biodiesel production. Methods Mol. Biol. 861:471–483 - PubMed
-
- Hudlicky T, Reed JW. 2009. Applications of biotransformations and biocatalysis to complexity generation in organic synthesis. Chem. Soc. Rev. 38:3117–3132 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

