Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin
- PMID: 23542919
- PMCID: PMC3680721
- DOI: 10.1152/ajpheart.00081.2013
Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin
Abstract
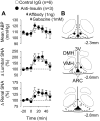
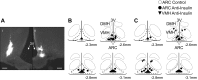
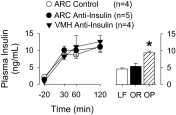
Accumulating evidence suggests that insulin acts within the hypothalamus to alter sympathetic nerve activity (SNA) and baroreflex function. Although insulin receptors are widely expressed across the hypothalamus, recent evidence suggests that neurons of the arcuate nucleus (ARC) play an important role in the sympathoexcitatory response to insulin. The purpose of the present study was to determine whether circulating insulin acts directly in the ARC to elevate SNA. In anesthetized male Sprague-Dawley rats (275-425 g), the action of insulin was neutralized by microinjection of an anti-insulin affibody (1 ng/40 nl). To verify the efficacy of the affibody, ARC pretreatment with injection of the anti-insulin affibody completely prevented the increase in lumbar SNA produced by ARC injection of insulin. Next, ARC pretreatment with the anti-insulin affibody attenuated the lumbar sympathoexcitatory response to intracerebroventricular injection of insulin. Third, a hyperinsulinemic-euglycemic clamp increased lumbar, but not renal, SNA in animals that received ARC injection of a control affibody. However, this sympathoexcitatory response was absent in animals pretreated with the anti-insulin affibody in the ARC. Injection of the anti-insulin affibody in the adjacent ventromedial hypothalamus did not alter the sympathoexcitatory response to insulin. The ability of the anti-insulin affibody to prevent the sympathetic effects of insulin cannot be attributed to a general inactivation or nonspecific effect on ARC neurons as the affibody did not alter the sympathoexcitatory response to ARC disinhibition by gabazine. Collectively, these findings suggest that circulating insulin acts within the ARC to increase SNA.
Keywords: blood pressure; lumbar; obesity; renal; sympathetic nerve activity.
Figures




Similar articles
-
Glucocorticoids attenuate the central sympathoexcitatory actions of insulin.J Neurophysiol. 2014 Nov 15;112(10):2597-604. doi: 10.1152/jn.00514.2014. Epub 2014 Sep 3. J Neurophysiol. 2014. PMID: 25185805 Free PMC article.
-
Sex differences in the sympathoexcitatory response to insulin in obese rats: role of neuropeptide Y.J Physiol. 2019 Mar;597(6):1757-1775. doi: 10.1113/JP277517. Epub 2019 Feb 3. J Physiol. 2019. PMID: 30628058 Free PMC article.
-
Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats.J Physiol. 2011 Apr 1;589(Pt 7):1643-62. doi: 10.1113/jphysiol.2011.205575. Epub 2011 Feb 7. J Physiol. 2011. PMID: 21300750 Free PMC article.
-
Role of the hypothalamic arcuate nucleus in cardiovascular regulation.Auton Neurosci. 2013 Apr;175(1-2):38-50. doi: 10.1016/j.autneu.2012.10.016. Epub 2012 Dec 19. Auton Neurosci. 2013. PMID: 23260431 Free PMC article. Review.
-
Central angiotensin modulation of baroreflex control of renal sympathetic nerve activity in the rat: influence of dietary sodium.Acta Physiol Scand. 2003 Mar;177(3):285-9. doi: 10.1046/j.1365-201X.2003.01074.x. Acta Physiol Scand. 2003. PMID: 12608998 Review.
Cited by
-
Activation of corticotropin-releasing factor receptors in the rostral ventrolateral medulla is required for glucose-induced sympathoexcitation.Am J Physiol Endocrinol Metab. 2014 Nov 15;307(10):E944-53. doi: 10.1152/ajpendo.00291.2014. Epub 2014 Sep 30. Am J Physiol Endocrinol Metab. 2014. PMID: 25269482 Free PMC article.
-
Neural Control of Non-vasomotor Organs in Hypertension.Curr Hypertens Rep. 2016 Apr;18(4):30. doi: 10.1007/s11906-016-0635-8. Curr Hypertens Rep. 2016. PMID: 26957306 Free PMC article. Review.
-
Insulin and sympathoexcitation: it is not all in your head.Diabetes. 2013 Aug;62(8):2654-5. doi: 10.2337/db13-0613. Diabetes. 2013. PMID: 23881194 Free PMC article. No abstract available.
-
Role of the carotid chemoreceptors in insulin-mediated sympathoexcitation in humans.Am J Physiol Regul Integr Comp Physiol. 2020 Jan 1;318(1):R173-R181. doi: 10.1152/ajpregu.00257.2019. Epub 2019 Nov 20. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31746629 Free PMC article. Clinical Trial.
-
Neuronal Networks in Hypertension: Recent Advances.Hypertension. 2020 Aug;76(2):300-311. doi: 10.1161/HYPERTENSIONAHA.120.14521. Epub 2020 Jun 29. Hypertension. 2020. PMID: 32594802 Free PMC article. Review.
References
-
- Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 50: 354–359, 2007 - PubMed
-
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation 106: 2533–2536, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

