A mitochondrial bioenergetic etiology of disease
- PMID: 23543062
- PMCID: PMC3614529
- DOI: 10.1172/JCI61398
A mitochondrial bioenergetic etiology of disease
Abstract
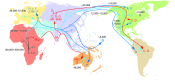
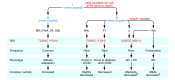
The classical Mendelian genetic perspective has failed to adequately explain the biology and genetics of common metabolic and degenerative diseases. This is because these diseases are primarily systemic bioenergetic diseases, and the most important energy genes are located in the cytoplasmic mitochondrial DNA (mtDNA). Therefore, to understand these "complex" diseases, we must investigate their bioenergetic pathophysiology and consider the genetics of the thousands of copies of maternally inherited mtDNA, the more than 1,000 nuclear DNA (nDNA) bioenergetic genes, and the epigenomic and signal transduction systems that coordinate these dispersed elements of the mitochondrial genome.
Figures

References
-
- Wallace DC, Lott MT, Procaccio V. Mitochondrial medicine: the mitochondrial biology and genetics of metabolic and degenerative diseases, cancer, and aging. In: Rimoin DL, Pyeritz RE, Korf BR, eds.Emery and Rimoin’s Principles and Practice of Medical Genetics. Philadelphia, Pennsylvania, USA: Churchill Livingstone Elsevier; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

