Relevance of the Core 70 and IL-28B polymorphism and response-guided therapy of peginterferon alfa-2a ± ribavirin for chronic hepatitis C of Genotype 1b: a multicenter randomized trial, ReGIT-J study
- PMID: 23543311
- PMCID: PMC3953545
- DOI: 10.1007/s00535-013-0785-2
Relevance of the Core 70 and IL-28B polymorphism and response-guided therapy of peginterferon alfa-2a ± ribavirin for chronic hepatitis C of Genotype 1b: a multicenter randomized trial, ReGIT-J study
Abstract
Background: We conducted a multicenter randomized clinical trial to determine the optimal treatment strategy against chronic hepatitis C virus (HCV) with genotype 1b and a high viral load (G1b/high).
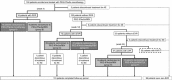
Methods: The study subjects included 153 patients with G1b/high. Patients were initially treated with PEG-IFNα-2a alone and then randomly assigned to receive different treatment regimens. Ribavirin (RBV) was administered to all patients with HCV RNA at week 4. Patients negative for HCV RNA at week 4 were randomly assigned to receive PEG-IFNα-2a (group A) or PEG-IFNα-2a/RBV (group B). Patients who showed HCV RNA at week 4 but were negative at week 12 were randomly assigned to receive weekly PEG-IFNα-2a (group C) or biweekly therapy (group D). Patients who showed HCV RNA at week 12 but were negative at week 24 were randomly assigned to receive PEG-IFNα-2a/RBV (group E) or PEG-IFNα-2a/RBV/fluvastatin (group F).
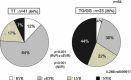
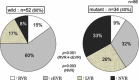
Results: Overall, the rate of sustained virological response (SVR) was 46 % (70/153). The total SVR rate in the group (A, D, and F) of response-guided therapy was significantly higher than that in the group (B, C, and E) of conventional therapy [70 % (38/54) versus 52 % (32/61), p = 0.049]. Although IL28-B polymorphism and Core 70 mutation were significantly associated with efficacy, patients with rapid virological response (RVR) and complete early virological response (cEVR) achieved high SVR rates regardless of their status of IL-28B polymorphism and Core 70 mutation.
Conclusion: In addition to knowing the IL-28B polymorphism and Core 70 mutation status, understanding the likelihood of virological response during treatment is critical in determining the appropriate treatment strategy.
Figures


Similar articles
-
Randomized clinical trial comparing high versus standard dose of ribavirin plus peginterferon alfa-2a in hepatitis C genotype 3 and high viral load. Dargen-3 study.Gastroenterol Hepatol. 2014 Jan;37(1):1-8. doi: 10.1016/j.gastrohep.2013.10.005. Epub 2013 Dec 17. Gastroenterol Hepatol. 2014. PMID: 24360571 Clinical Trial.
-
Cost-efficacy analysis of peginterferon alfa-2b plus ribavirin compared with peginterferon alfa-2a plus ribavirin for the treatment of chronic hepatitis C.J Manag Care Pharm. 2005 Oct;11(8):687-94. doi: 10.18553/jmcp.2005.11.8.687. J Manag Care Pharm. 2005. PMID: 16194133 Free PMC article.
-
Efficacy and safety of peg-IFN alfa-2a with ribavirin for the treatment of HCV/HIV coinfected patients who failed previous IFN based therapy.J Clin Virol. 2007 Jan;38(1):32-8. doi: 10.1016/j.jcv.2006.09.009. Epub 2006 Oct 24. J Clin Virol. 2007. PMID: 17064957 Clinical Trial.
-
Viral hepatitis C gets personal--the value of human genomics to public health.Public Health Genomics. 2013;16(4):192-7. doi: 10.1159/000352014. Epub 2013 Jul 11. Public Health Genomics. 2013. PMID: 23859951 Free PMC article. Review.
-
Response-guided and -unguided treatment of chronic hepatitis C.Liver Int. 2012 Feb;32 Suppl 1:64-73. doi: 10.1111/j.1478-3231.2011.02713.x. Liver Int. 2012. PMID: 22212575 Review.
Cited by
-
MHC class I-related chain B gene polymorphism is associated with virological response to pegylated interferon plus ribavirin therapy in patients with chronic hepatitis C infection.Biomed Rep. 2015 Mar;3(2):247-253. doi: 10.3892/br.2014.406. Epub 2014 Dec 17. Biomed Rep. 2015. PMID: 26075078 Free PMC article.
-
Recent Advances in Antiviral Therapy for Chronic Hepatitis C.Mediators Inflamm. 2016;2016:6841628. doi: 10.1155/2016/6841628. Epub 2016 Jan 31. Mediators Inflamm. 2016. PMID: 27022210 Free PMC article. Review.
-
Hepatitis C Genotype Prevalence in Monastir Region, Tunisia: Correlation between 5' Untranslated Region (5'UTR), Non-structural 5B (NS5B), and Core Sequences in HCV Subtyping.Curr Microbiol. 2016 Sep;73(3):324-334. doi: 10.1007/s00284-016-1064-2. Epub 2016 May 17. Curr Microbiol. 2016. PMID: 27189386
-
Predictive value of the IFNL4 polymorphism on outcome of telaprevir, peginterferon, and ribavirin therapy for older patients with genotype 1b chronic hepatitis C.J Gastroenterol. 2014 Dec;49(12):1548-56. doi: 10.1007/s00535-013-0924-9. Epub 2013 Dec 21. J Gastroenterol. 2014. PMID: 24362944 Clinical Trial.
-
The Prevalence of Hepatitis C Virus Core Amino Acid 70 Substitution and Genotypes of Polymorphisms Near the IFNL3 Gene in Iranian Patients With Chronic Hepatitis C.Hepat Mon. 2016 May 3;16(6):e37011. doi: 10.5812/hepatmon.37011. eCollection 2016 Jun. Hepat Mon. 2016. PMID: 27630727 Free PMC article.
References
-
- Kuboki M, Iino S, Okuno T, Omata M, et al. Peginterferon a-2a (40 KD) plus ribavirin for the treatment of chronic hepatitis C in Japanese patients. J Gastroenterol Hepatol. 2007;22:645–652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

