Megestrol acetate for treatment of anorexia-cachexia syndrome
- PMID: 23543530
- PMCID: PMC6418472
- DOI: 10.1002/14651858.CD004310.pub3
Megestrol acetate for treatment of anorexia-cachexia syndrome
Abstract
Background: This is an updated version of a previously published review in The Cochrane Library (2005, Issue 2) on 'Megestrol acetate for the treatment of anorexia-cachexia syndrome'. Megestrol acetate (MA) is currently used to improve appetite and to increase weight in cancer-associated anorexia. In 1993, MA was approved by the US Food and Drug Administration for the treatment of anorexia, cachexia or unexplained weight loss in patients with AIDS. The mechanism by which MA increases appetite is unknown and its effectiveness for anorexia and cachexia in neoplastic and AIDS (acquired immunodeficiency syndrome) patients is under investigation.
Objectives: To evaluate the efficacy, effectiveness and safety of MA in palliating anorexia-cachexia syndrome in patients with cancer, AIDS and other underlying pathologies.
Search methods: We sought studies through an extensive search of electronic databases, journals, reference lists, contact with investigators and other search strategies outlined in the methods. The most recent search for this update was carried out in May 2012.
Selection criteria: Studies were included in the review if they assessed MA compared to placebo or other drug treatments in randomised controlled trials of patients with a clinical diagnosis of anorexia-cachexia syndrome related to cancer, AIDS or any other underlying pathology.
Data collection and analysis: Two independent review authors conducted data extraction and evaluated methodological quality. We performed quantitative analyses using appetite and quality of life as a dichotomous variable, and analysed weight gain as continuous and dichotomous variables.
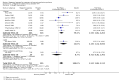
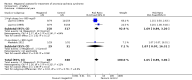
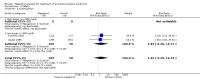
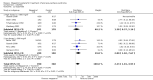
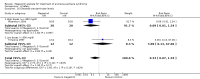
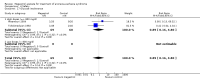
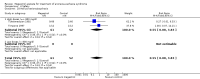
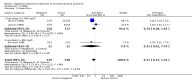
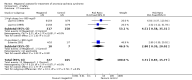
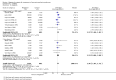
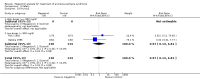
Main results: We included 35 trials in this update, the same number but not the same trials as in the previous version of the review. The trials comprised 3963 patients for effectiveness and 3180 for safety. Sixteen trials compared MA at different doses with placebo, seven trials compared different doses of MA with other drug treatments and 10 trials compared different doses of MA. Meta-analysis showed a benefit of MA compared with placebo, particularly with regard to appetite improvement and weight gain in cancer, AIDS and other underlying conditions, and lack of benefit in the same patients when MA was compared to other drugs. There was insufficient information to define the optimal dose of MA, but higher doses were more related to weight improvement than lower doses. Quality of life improvement in patients was seen only when comparing MA versus placebo but not other drugs in both subcategories: cancer and AIDS. Oedema, thromboembolic phenomena and deaths were more frequent in the patients treated with MA. More than 40 side effects were studied.
Authors' conclusions: This review shows that MA improves appetite and is associated with slight weight gain in cancer, AIDS and in patients with other underlying pathology. Despite the fact that these patients are receiving palliative care they should be informed of the risks involved in taking MA.
Conflict of interest statement
No one involved in this review has any conflict of interest.
Figures


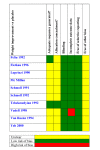
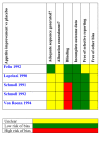
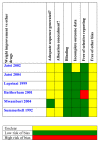
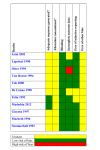













































































Update of
-
Megestrol acetate for the treatment of anorexia-cachexia syndrome.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004310. doi: 10.1002/14651858.CD004310.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2013 Mar 28;(3):CD004310. doi: 10.1002/14651858.CD004310.pub3. PMID: 15846706 Updated.
References
References to studies included in this review
Batterham 2001 {published data only}
-
- Batterham M, Garsia R. Randomised prospective medium term comparison of megestrol acetate, nandrolone decanoate and dietary therapy alone for HIV associated weight loss [abstract]. Australasian Society for HIV Medicine 1997;9:91.
-
- Batterham MJ, Garsia R. A comparison of megestrol acetate, nandrolone decanoate and dietary counselling for HIV associated weight loss. International Journal of Andrology 2001;24(4):232‐40. - PubMed
Beller 1997 {published data only}
-
- Beller E, Tattersall M. Improved quality of life with megestrol acetate in patients with endocrine‐insensitive advanced cancer: a randomised placebo‐controlled trial. Annals of Oncology 1997;8:277‐83. - PubMed
Casado 2008 {unpublished data only}
-
- Casado Herráez A. Phase II randomized study of two dose levels of megestrol acetate versus placebo for the treatment of anorexia and cachexia in patients with locoregional advanced or metastatic cancer [Estudio de fase II randomizado de dos niveles posológicos de acetato de Megestrol versus placebo para el tratamiento de la anorexia y la caquexia en pacientes con cáncer locoregional avanzado o metastásico. (Tesis de Doctorado ‐ Universidad Complutense Madrid)]. Available from http://eprints.ucm.es/8119/1/T30450.pdf 2008 (accessed 24 December 2011).
De Conno 1998 {published data only}
-
- Conno F, Martini C. Megestrol acetate for anorexia in patients with far‐advanced cancer: a double‐blind controlled clinical trial. European Journal of Cancer 1998;34:1705‐9. - PubMed
Eubanks 2002 {published data only}
-
- Eubanks V, Koppersmith N. Effects of megestrol acetate on weight gain, body composition, and pulmonary function in patients with cystic fibrosis. Journal of Pediatrics 2002;140:439‐44. - PubMed
Feliu 1992 {published data only}
-
- Feliu J, Gonzalez‐Baron M. Usefulness of megestrol acetate in cancer cachexia and anorexia. A placebo controlled study. American Journal of Clinical Oncology 1992;15:436‐40. - PubMed
-
- Gonzalez Baron M, Feliu J, Zamora P, Artal A, Garrido P, Chacon I. Usefulness of megestrol acetate on cancer cachexia: a double blind randomized study. Annals of Oncology 1990;1:29‐31.
Fietkau 1996 {published data only}
-
- Fietkau R, Riepl M. Supportive treatment with megestrol acetate during radio (chemo) therapy. A randomised trial. Strahlentherapie und Onkologie 1996;172:162‐8. - PubMed
-
- Fietkau R, Riepl M, Kettner H, Hinke A, Sauer R. Supportive use of megestrol acetate in patients with head and neck cancer during radio(chemo)therapy. European Journal of Cancer 1997;33:75‐9. [CN‐00137738] - PubMed
Gambardella 1998 {published data only}
-
- Gambardella A, Pesce L, Bolognino P, Lombardi G, Barbieri M, Rinaldi C. Megestrol acetate prevents cachexia in elderly cancer patient. Annals of Oncology 1998;9(Suppl 3):72. [CN‐00305911]
-
- Gambardella A, Pesce L, Lammardo AM, Lombardi G, Barbagallo M, Tirelli P, et al. New factors involvement in elderly cancer patients with anorexia/cachexia syndrome role of megestrol acetate. Annals of Oncology 2000;9 Suppl 2:22.
Gebbia 1996 {published data only}
Giacosa 1997 {published data only}
-
- Giacosa A, Frascio F, Sukkar SG, Costantini M, Baracco G, Capelli M. Changes of nutritional and psychological status after megestrol acetate treatment of cancer cachexia. Rivista Italiana Di Nutrizione Parenterale Ed Enterale. 1997;15:20‐3.
Heckmayr 1992 {published data only}
-
- Heckmayr M, Gatzemeier U. Treatment of cancer weight loss in patients with advanced lung cancer. Oncology 1992;49(Suppl 2):32‐4. - PubMed
Herrejon 2011 {published data only}
-
- Herrejon A, Catalan P, Palop J, Inchaurraga I, Blanquer R, Lopez A. Effect of 8‐week 320 mg megestrol acetate daily administration in severe COPD and weight loss [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A4484.
-
- Herrejon A, Palop J, Inchaurraga I, Lopez A, Bañuls C, Hernandez A, et al. Low doses of megestrol acetate increase weight and improve nutrition status in patients with severe chronic obstructive pulmonary disease and weight loss. Medicina Clinica 2011;137:193‐8. - PubMed
Jatoi 2002 {published data only}
-
- Jatoi A, Windschitl HE. Dronabinol versus megestrol acetate versus combination for cancer‐associated anorexia: A North Central Cancer Treatment Group Study. Journal of Clinical Oncology 2002;20:567‐73. - PubMed
Jatoi 2004 {published data only}
-
- Jatoi A, Rowland K, Loprinzi CL, Sloan JA, Dakhil SR, MacDonald N, et al. An eicosapentaenoic acid supplement versus megestrol acetate versus both for patients with cancer‐associated wasting: a North Central Cancer Treatment Group and National Cancer Institute of Canada collaborative effort. Journal of Clinical Oncology 2004;22:2469‐76. - PubMed
Lesser 2006 {published data only}
-
- Lesser GJ, Case D, Sharp S, Choksi J, Miller A, Atkins J, et al. A phase III randomized study comparing the effects of oxandrolone (Ox) and megestrol acetate (Meg) on weight (wt), lean body mass (LBM) and quality of life (QOL) in solid tumor patients (pts) receiving chemotherapy (chemo). Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I 2006;24(18S (June 20 Suppl)):18546.
Loprinzi 1990b {published data only}
-
- Loprinzi CL, Ellison NM. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. Journal of the National Cancer Institute 1990;82:1127‐32. - PubMed
Loprinzi 1994 {published data only}
-
- Loprinzi CL, Bernath AM. Phase III evaluation of 4 doses of megestrol acetate for patients with cancer anorexia and/or cachexia. Oncology 1994;51:2‐7. - PubMed
-
- Loprinzi CL, Michalak JC, Schaid DJ, Mailliard JA, Athmann LM, Goldberg RM, et al. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. Journal of Clinical Oncology 1993;11:762‐7. [CN‐00092689] - PubMed
Loprinzi 1999a {published data only}
-
- Loprinzi CL, Kugler J, Sloan J, Mailliard J, Krook J, Wilwerding M, et al. Phase III randomized comparison of megestrol acetate, dexamethasone, and fluoxymesterone for the treatment of cancer anorexia/cachexia [Meeting abstract no 167]. ASCO Annual Meeting. 1997.
-
- Loprinzi CL, Kugler JW. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. Journal of Clinical Oncology 1999;17:3299‐306. - PubMed
Loprinzi 1999b {published data only}
-
- Loprinzi CL, Kugler J, Sloan J, Mailliard J, Krook J, Wilwerding M, et al. Phase III randomized comparison of megestrol acetate, dexamethasone, and fluoxymesterone for the treatment of cancer anorexia/cachexia [Meeting abstract no 167]. ASCO Annual Meeting. 1997.
-
- Loprinzi CL, Kugler JW. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. Journal of Clinical Oncology 1999;17:3299‐306. - PubMed
Macbeth 1994 {published and unpublished data}
-
- Macbeth FR, Grgeor A, Cott. A randomised study of megestrol acetate (MA) and prednisolone (P) for anorexia and weight loss in patients with lung cancer. Lung Cancer 1994;1 Suppl 1:88.
Madeddu 2012 {published data only}
-
- Madeddu C, Dessì M, Panzone F, Serpe R, Antoni G, Cau MC, et al. Randomized phase III clinical trial of a combined treatment with carnitine + celecoxib ± megestrol acetate for patients with cancer‐related anorexia/cachexia syndrome. Clinical Nutrition 2012;31:176‐82. - PubMed
McMillan 1994 {published data only}
-
- McMillan DC, Simpson JM. Effect of megestrol acetate on weight loss, body composition and blood screen of gastrointestinal cancer patients. Clinical Nutrition 1994;13:85‐9. - PubMed
Mwamburi 2004 {published data only}
-
- Mwamburi DM, Gerrior J, Wilson IB, Chang H, Scully E, Saboori S, et al. Comparing megestrol acetate therapy with oxandrolone therapy for HIV‐related weight loss: similar results in 2 months. Clinical Infectious Diseases 2004;38:895‐902. - PubMed
Oster 1994 {published data only}
-
- Oster MH, Enders SR. Megestrol acetate in patients with AIDS and cachexia. Annals of Internal Medicine 1994;121:400‐8. - PubMed
Sancho‐Cuesta 1993 {published data only}
-
- Sancho‐Cuesta JF. Megestrol acetate and weight loss in advanced cancer [Abstract no: 1141]. European Journal of Cancer 1993;29A:S204.
Schmoll 1991 {published data only}
-
- Schmoll E, Wilke H, Thole R, Preusser P, Wildfang I, Schmoll HJ. Megestrol acetate in cancer cachexia. Seminars in Oncology 1991;18(1 Suppl 2):32‐4. [CN‐00072963] - PubMed
Schmoll 1992 {published data only}
-
- Schmoll E. Risks and benefits of various therapies for cancer anorexia. Oncology 1992;49:43‐5. - PubMed
Summerbell 1992 {published data only}
-
- Summerbell CD, Youle M, McDonald V, Catalan J, Gazzard BG. Megestrol acetate vs cyproheptadine in the treatment of weight loss associated with HIV infection. International Journal of STD and AIDS 1992;3:278‐80. - PubMed
Tchekmedyian 1992 {published data only}
-
- Tchekmedyian NS, Hickman M. Megestrol acetate in cancer anorexia and weight loss. Cancer 1992;69:1268‐74. - PubMed
Timpone 1997 {published data only}
-
- Timpone JG, Wright DJ. The safety and pharmacokinetics of single‐agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome. AIDS Research and Human Retroviruses 1997;13:305‐15. - PubMed
Ulutin 2002 {published data only}
-
- Ulutin HC, Arpaci F, Pak Y. Megestrol acetate for cachexia and anorexia in advanced non‐small cell lung cancer: a randomized study comparing two different doses. Tumori 2002;88:277‐80. - PubMed
Vadell 1998 {published data only}
-
- Segui MA, Vadell C, Gimenez‐Arnau JM, Morales S, Cirera L, Bestit I, et al. Double‐blind randomized trial for the treatment of cancer related cachexia: comparison of placebo vs two different doses of megestrol acetate [Abstract 99]. Proceedings of the American Society of Clinical Oncology 1996;15:529.
-
- Vadell C, Segui MA. Anticachectic efficacy of megestrol acetate at different doses and versus placebo in patients with neoplastic cachexia. American Journal of Clinical Oncology 1998;21:347‐51. - PubMed
Von Roenn 1994 {published data only}
-
- Roenn JH, Armstrong D. Megestrol acetate in patients with AIDS‐related cachexia. Annals of Internal Medicine 1994;121:393‐9. - PubMed
Wanke 2007 {published data only}
-
- Cilla DD, Gutierrez JL, Kristensen A, Kramer LD. The safety of megestrol acetate concentrated suspension (MA‐CS) and megestrol acetate oral suspension (MA‐OS) in a pilot study in patients with HIV‐associated unintended weight loss (UWL) [Abstract]. Blood 2006;108:43.
-
- Wanke C, Gutierrez J, Kristensen A, MacEarchern, L. Safety and efficacy of two preparations of megestrol acetate in HIV‐infected individuals with weight loss in Africa, India, and the United States. Journal of Applied Research 2007;7:206‐16.
Weisberg 2002 {published data only}
-
- Weisberg J, Wanger J. Megestrol acetate stimulates weight gain and ventilation in underweight COPD patients. Chest 2002;121(4):1070‐8. - PubMed
Yeh 2000 {published data only}
-
- Yeh SS, Lovitt S, Schuster MW. Usage of megestrol acetate in the treatment of anorexia‐cachexia syndrome in the elderly. Journal of Nutrition, Health and Aging 2009;13:448‐54. - PubMed
-
- Yeh SS, Wu SY. Improvement in quality of life measures and stimulation of weight gain after treatment with megestrol acetate oral suspension in geriatric cachexia: results of a double‐blind, placebo‐controlled study. Journal of the American Geriatrics Society 2000;48(5):485‐92. - PubMed
-
- Yeh SS, Wu SY, Levine DM, Parker TS, Olson JS, Stevens MR, et al. The correlation of cytokine levels with body weight after megestrol acetate treatment in geriatric patients. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2001;56:M48‐54. - PubMed
References to studies excluded from this review
Aguilera 2001 {published data only}
-
- Aguilera A, Selgas R, Diez JJ, Bajo MA, Codoceo R, Alvarez V. Anorexia in end‐stage renal disease: pathophysiology and treatment. Expert Opinion on Pharmacotherapy 2001;2(11):1825‐38. - PubMed
Aisner 1988 {published data only}
-
- Aisner J, Tchekmedyian NS, Tait N, Parnes H, Novak M. Studies of high‐dose megestrol acetate: potential applications in cachexia. Seminars in Oncology 1988;15(2 Suppl 1):68‐75. - PubMed
Anonymous 1995 {published data only}
-
- Anonymous. Megestrol found effective in two trials. AIDS Patient Care 1995;9(1):41.
Ansfield 1982 {published data only}
-
- Ansfield FJ, Kallas GJ, Singson JP. Clinical results with megestrol acetate in patients with advanced carcinoma of the breast. Surgery, Gynecology & Obstetrics 1982;155(6):888‐90. - PubMed
Argiles 2007 {published data only}
-
- Argiles JM, Lopez‐Soriano FJ, Busquets S. Emerging drugs for cancer cachexia. Expert Opinion on Emerging Drugs 2007;12(4):555‐70. - PubMed
Argiles 2008 {published data only}
-
- Argiles JM, Lopez‐Soriano FJ, Busquets S. Novel approaches to the treatment of cachexia. Drug Discovery Today 2008;13(1‐2):73‐8. - PubMed
Argiles 2010 {published data only}
Behl 2007 {published data only}
-
- Behl D, Jatoi A. Pharmacological options for advanced cancer patients with loss of appetite and weight. Expert Opinion on Pharmacotherapy 2007;8(8):1085‐90. - PubMed
Bossola 2006 {published data only}
-
- Bossola M, Tazza L, Giungi S, Luciani G. Anorexia in hemodialysis patients: an update. Kidney International 2006;70(3):417‐22. - PubMed
Bossola 2009 {published data only}
-
- Bossola M, Tazza L, Luciani G. Mechanisms and treatment of anorexia in end‐stage renal disease patients on hemodialysis. Journal of Renal Nutrition 2009;19(1):2‐9. - PubMed
Bruera 1990 {published data only}
-
- Bruera E, McMillan K. A controlled trial of megestrol acetate on appetite, caloric intake, nutritional status, and other symptoms in patients with advanced cancer. Cancer 1990;66:1279‐82. - PubMed
Bruera 1992a {published data only}
-
- Bruera E. Clinical management of anorexia and cachexia in patients with advanced cancer. Oncology 1992;49 Suppl 2:35‐42. - PubMed
Bruera 1998 {published data only}
-
- Bruera E, Scott E. Effectiveness of megestrol acetate in patients with advanced cancer: a randomized, double‐blind, crossover study. Cancer Prevention and Control 1998;2(2):74‐8. - PubMed
Bruera 1998a {published data only}
-
- Bruera E. Pharmacological treatment of cachexia: any progress? [Review]. Supportive Care in Cancer 1998;6(2):109‐13. - PubMed
Cardona 2006 {published data only}
-
- Cardona D. Pharmacological therapy of cancer anorexia‐cachexia. Nutricion Hospitalaria 2006;21:Suppl 26. - PubMed
Carroll 2007 {published data only}
-
- Carroll JK, Kohli S, Mustian KM, Roscoe JA, Morrow GR. Pharmacologic treatment of cancer‐related fatigue. Oncologist 2007;12(Suppl 1):43‐51. - PubMed
Cat 1994 {published data only}
-
- Cat LK, Coleman RL. Treatment for HIV wasting syndrome. Annals of Pharmacotherapy 1994;28(5):595‐7. - PubMed
Celik 2009 {published data only}
-
- Celik T, Iyisoy A, Gundogdu F, Isik E. Chronic pulmonary cachexia syndrome: the role of anorexia. International Journal of Cardiology 2009;132(3):432‐3. - PubMed
Chen 1997 {published data only}
-
- Chen HC, Leung SW, Wang CJ, Sun LM, Fang FM, Hsu JH. Effect of megestrol acetate and prepulsid on nutritional improvement in patients with head and neck cancers undergoing radiotherapy. Radiotherapy and Oncology 1997;43:75‐9. - PubMed
Chlebowski 1996 {published data only}
-
- Chlebowski RT, Palomares MR, Lillington L, Grosvenor M. Recent implications of weight loss in lung cancer management. Nutrition 1996;12(1 Suppl):S43‐7. - PubMed
Costero 2004 {published data only}
-
- Costero O, Bajo MA, Peso G, Gil F, Aguilera A, Ros S, et al. Treatment of anorexia and malnutrition in peritoneal dialysis patients with megestrol acetate. Advances in Peritoneal Dialysis 2004;20:209‐21. - PubMed
Cruz 1990 {published data only}
-
- Cruz JM, Muss HB, Brockschmidt JK, Evans GW. Weight changes in women with metastatic breast cancer treated with megestrol acetate: a comparison of standard versus high‐dose therapy. Seminars in Oncology 1990;17:63‐7. - PubMed
Cuerda 1998 {published data only}
-
- Cuerda Compes M, Breton Lesmes I, Camblor Alvarez M, Garcia Peris P. Pharmacological modulation of the appetite. Nutricion Hospitalaria 1998;13(2):69‐75. - PubMed
Desport 2000 {published data only}
-
- Desport JC, Blanc‐Vincent MP, Gory‐Delabaere G, Bachmann P, Beal J, Benamouzig R, et al. Standards, Options and Recommendations (SOR) for the use of appetite stimulants in oncology. Work group. Federation of the French Cancer Centres (FNCLCC). Bulletin du Cancer 2000;87(4):315‐28. - PubMed
Elovic 2000 {published data only}
-
- Elovic E. Pharmacological therapeutics in nutritional management. Journal of Head Trauma Rehabilitation 2000;15(3):962‐4. - PubMed
Erkurt 2000 {published data only}
-
- Erkurt E, Erkisi M, Tunali C. Supportive treatment in weight‐losing cancer patients due to the additive adverse effects of radiation treatment and/or chemotherapy. Journal of Experimental & Clinical Cancer Research 2000;19:431‐9. - PubMed
Farmer 2005 {published data only}
-
- Farmer M, Case D, Lesser G, Monitto D, Smathers S, May B, et al. A phase III, double blind, placebo‐controlled, prospective randomized trial on the effect of megestrol acetate on weight and health related quality of life in lung cancer and head and neck cancer patients receiving curative radiation therapy. Proceedings of the American Society for Therapeutic Radiology and Oncology, 47th.Annual Meeting; 2005 Oct 16‐19; Denver, Colorado, USA. International Journal of Radiation Oncology Biology Physics 2005;63:S77‐8.
Farrar 1999 {published data only}
-
- Farrar DJ. Megestrol acetate: promises and pitfalls [Review]. Aids Patient Care and STDS 1999;13(3):149‐52. - PubMed
Fearon 2002 {published data only}
-
- Fearon KCH, Moses AGW. Cancer cachexia. International Journal of Cardiology 2002;85(1):73‐81. - PubMed
Fossati 1998 {published data only}
-
- Fossati R, Confalonieri C, Torri V, Ghislandi E, Penna A, Pistotti V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. Journal of Clinical Oncology 1998;16(10):3439‐60. - PubMed
Fox 2009 {published data only}
-
- Fox CB, Treadway AK, Blaszczyk AT, Sleeper RB. Megestrol acetate and mirtazepine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy 2009;29(4):383‐97. - PubMed
Freyer 1996 {published data only}
-
- Freyer G, Catimel G, Merrouche Y. Synthetic progestational hormones in the treatment of neoplastic cachexia. Revue de Medecine Interne 1996;17(1):79‐84. - PubMed
Freyer 1996a {published data only}
-
- Freyer G, Catimel G, Merrouche Y. Progesterone derivatives in cancer cachexia. Revue de Medecine Interne 1996;17(1):79‐84. - PubMed
Gaducci 2001 {published data only}
-
- Gadducci A, Cosio S, Fanucchi A, Genazzani AR. Malnutrition and cachexia in ovarian cancer patients: pathophysiology and management. Anticancer Research 2011;21(4B):2941‐7. - PubMed
Garg 2010 {published data only}
-
- Garg S, Yoo J, Winquist E. Nutritional support for head and neck cancer patients receiving radiotherapy: a systematic review [Review]. Supportive Care in Cancer 2010;18(6):667‐77. - PubMed
Gullett {published data only}
Hanson 2011 {published data only}
Haren 2006 {published data only}
-
- Haren MT, Kim MJ, Kevorkian RT. Megestrol acetate for geriatric anorexia/cachexia. Journal of the American Geriatrics Society 2006;54(1):172‐3. - PubMed
Hellerstein 1990 {published data only}
-
- Hellerstein MK, Kahn J, Mudie H, Viteri F. Current approach to the treatment of human immunodeficiency virus‐associated weight loss: pathophysiologic considerations and emerging management strategies. Seminars in Oncology 1990;17(6 Suppl 9):33. - PubMed
Hoffman 1998 {published data only}
-
- Hoffman KR, Green NJ. Megestrol acetate (Megace) to prevent cachexia and marasmus in elderly cancer patients (Meeting abstract no 233). ASCO Annual Meeting. 1998.
Inui 2002 {published data only}
-
- Inui A. Cancer anorexia‐cachexia syndrome: current issues in research and management. CA: A Cancer Journal for Clinicians 2002;52:72‐91. - PubMed
Jatoi 2001 {published data only}
-
- Jatoi A, Loprinzi CL. An update: cancer‐associated anorexia as a treatment target. Current Opinion in Clinical Nutrition and Metabolic Care 2001;4(3):179‐82. - PubMed
Kalantar‐Zadeh 2008 {published data only}
Karcic 2002 {published data only}
-
- Karcic E, Philpot C, Morley JE. Treating malnutrition with megestrol acetate: literature review and review of our experience. Journal of Nutrition, Health, and Aging 2002;6:191‐200. - PubMed
Khojasteh 1996 {published data only}
-
- Khojasteh A, Khojasteh S, Reynolds R. Combined nortriptyline + megestrol acetate regimen for cancer anorexia‐cachexia syndrome [abstract]. Proceedings of the American Society of Clinical Oncology 1996;15:451.
Krznaric 2007 {published data only}
-
- Krznaric Z, Juretic A, Samija M, Dintinjana RD, Vrdoljak E, Samarzija M, et al. Croatian guidelines for use of eicosapentaenoic acid and megestrol acetate in cancer cachexia syndrome [Croatian]. Lijecnicki Vjesnik 2007;129:381‐6. - PubMed
Kumar 2010 {published data only}
Lai 1994 {published data only}
-
- Lai YL, Fang FM, Yeh CY. Management of anorexic patients in radiotherapy: a prospective randomized comparison of megestrol and prednisolone. Journal of Pain and Symptom Management 1994;9:265‐8. - PubMed
Lelli 2003 {published data only}
-
- Lelli G, Montanari M, Gilli G, Scapoli D, Antonietti C, Scapoli D. Treatment of the cancer anorexia‐cachexia syndrome: a critical reappraisal. Journal of Chemotherapy 2003;15:220‐5. - PubMed
Lesniak 2008 {published data only}
-
- Lesniak W, Bala M, Jaeschke R, Krzakowski M. Effects of megestrol acetate in patients with cancer anorexia‐cachexia syndrome‐‐a systematic review and meta‐analysis. Polskie Archiwum Medycyny Wewnętrznej 2008;118:636‐44. - PubMed
Loprinzi 1992 {published data only}
-
- Loprinzi CL, Goldberg RM, Burnham NL. Cancer‐associated anorexia and cachexia. Implications for drug therapy. Drugs 1992;43:499‐506. - PubMed
Loprinzi 1992a {published data only}
-
- Loprinzi CL, Johnson PA, Jensen M. Megestrol acetate for anorexia and cachexia. Oncology 1992;49 Suppl 2:46‐9. - PubMed
Loprinzi 1993 {published data only}
-
- Loprinzi CL, Schaid DJ, Dose AM, Burnham NL, Jensen MD. Body‐composition changes in patients who gain weight while receiving megestrol acetate. Journal of Clinical Oncology 1993;11:152‐4. - PubMed
Loprinzi 1995 {published data only}
-
- Loprinzi CL. Management of cancer anorexia/cachexia. Supportive Care in Cancer 1995;3:120‐2. - PubMed
Mak 2007 {published data only}
-
- Mak RH, Cheung W. Therapeutic strategy for cachexia in chronic kidney disease. Current Opinion in Nephrology and Hypertension 2007;16:542‐6. - PubMed
Malone 2005 {published data only}
-
- Malone M. Medications associated with weight gain. Annals of Pharmacotherapy 2005;39:2046‐55. - PubMed
Maltoni 2001a {published data only}
-
- Maltoni M, Nanni O, Scarpi E, Rossi D, Serra P, Amadori D. High‐dose progestins for the treatment of cancer anorexia‐cachexia syndrome: a systematic review of randomised clinical trials. Annals of Oncology 2001;12:289‐300. - PubMed
Mantovani 1998a {published data only}
-
- Mantovani G, Maccio A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Critical Reviews in Oncogenesis 1998;9:99‐106. - PubMed
Mantovani 2001 {published data only}
-
- Mantovani G, Maccio A, Massa E, Madeddu C. Managing cancer‐related anorexia/cachexia. Drugs 2001;61:499‐514. - PubMed
Mantovani 2008 {published data only}
-
- Mantovani G, Macciò A, Madeddu C, Gramignano G, Serpe R, Massa E, et al. Randomized phase III clinical trial of five different arms of treatment for patients with cancer cachexia: interim results. Nutrition 2008;24:305‐13. - PubMed
Mantovani 2010 {published data only}
Marchand 2000 {published data only}
-
- Marchand V, Baker SS. Randomized, double‐blind, placebo‐controlled pilot trial of megestrol acetate in malnourished children with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2000;31:264‐9. - PubMed
Mateen 2006 {published data only}
-
- Mateen F, Jatoi A. Megestrol acetate for the palliation of anorexia in advanced, incurable cancer patients. Clinical Nutrition 2006;25:711‐5. - PubMed
McHugh 2011 {published data only}
-
- McHugh ME, Miller‐Saultz D. Assessment and management of gastrointestinal symptoms in advanced illness. Primary Care 2011;38:225‐46. - PubMed
McMillan 1999 {published data only}
McQuellon 2002 {published data only}
-
- McQuellon RP, Moose DB, Russell GB, Case LD, Greven K, Stevens M, et al. Supportive use of megestrol acetate (Megace) with head/neck and lung cancer patients receiving radiation therapy. International Journal of Radiation Oncology, Biology, Physics 2002;52:1180‐5. - PubMed
Monfared 2009 {published data only}
-
- Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. Journal of Renal Nutrition 2009;19:167‐71. - PubMed
Morán 1998 {published data only}
-
- Morán S, Ortega R, Garcia G, Uribe M. Megestrol acetate improve appetite and induce anti‐catabolic effect in post‐hepatitis C cirrhotic patients. A double‐blind placebo‐controlled clinical trial [Abstract 80]. Hepatology 1998;28:609A.
Morley 2002 {published data only}
-
- Morley JE. Orexigenic and anabolic agents. Clinics in Geriatric Medicine 2002;18:853‐66. - PubMed
Mulligan 2007 {published data only}
-
- Mulligan K, Zackin R, Von‐Roenn JH, Chesney MA, Egorin MJ, Sattler FR, et al. Testosterone supplementation of megestrol therapy does not enhance lean tissue accrual in men with human immunodeficiency virus‐associated weight loss: a randomized, double‐blind, placebo‐controlled, multicenter trial. Journal of Clinical Endocrinology and Metabolism 2007;92:563‐70. - PubMed
Muss 1990 {published data only}
-
- Muss HB, Case LD, Capizzi RL, Cooper MR, Cruz J, Jackson D, et al. High‐ versus standard‐dose megestrol acetate in women with advanced breast cancer: a phase III trial of the Piedmont Oncology Association. Journal of Clinical Oncology 1990;8:1797‐805. - PubMed
Navari 2010 {published data only}
-
- Navari RM, Brenner MC. Treatment of cancer‐related anorexia with olanzapine and megestrol acetate: a randomized trial. Supportive Care in Cancer 2010;18:951‐6. - PubMed
Nelson 2002 {published data only}
-
- Nelson KA, Walsh D, Hussein M. A phase II study of low‐dose megestrol acetate using twice‐daily dosing for anorexia in nonhormonally dependent cancer. American Journal of Hospice and Palliative Care 2002;19:206‐10. - PubMed
Osoba 1994 {published data only}
-
- Osoba D, Murray N, Gelmon K, Karsai H, Knowling MA, Shah A, et al. Phase II trial of megestrol in the supportive care of patients receiving dose‐intensive chemotherapy. Oncology 1994;8:43‐9. - PubMed
Pardo 2003a {published data only}
-
- Pardo J, Mena AM, Montsech L. Megestrol acetate for anorexia in lung cancer patients undergoing radiation therapy. A randomized trial comparing the efficacy of two different doses in 130 patients [Abstract 3076]. Proceedings of the American Society of Clinical Oncology 2003;22:765.
Pardo 2006 {published data only}
-
- Pardo J, Mena A, Monleon A, Macias V, Sole J, Hernandez M, et al. Reversal of anorexia with megestrol acetate (MA): impact on quality of life (QoL) in non‐metastatic lung cancer patients (pts) undergoing radiation therapy. Journal of Clinical Oncology 2006;24:18613.
Pascual 2004 {published data only}
-
- Pascual Lopez A, Figuls M, Urrutia Cuchi G, Berenstein EG, Almenar Pasies B, Balcells Alegre M, et al. Systematic review of megestrol acetate in the treatment of anorexia‐cachexia syndrome. Journal of Pain and Symptom Management 2004;27:360‐9. - PubMed
Pruthi 2007 {published data only}
-
- Pruthi S, Boughey JC, Brandt KR, Degnim AC, Dy GK, Goetz MP, et al. A multidisciplinary approach to the management of breast cancer, part 2: Therapeutic considerations. Mayo Clinic Proceedings 2007;82:1131‐40. - PubMed
Reuben 2005 {published data only}
-
- Reuben DB, Hirsch SH, Zhou K, Greendale GA. The effects of megestrol acetate suspension for elderly patients with reduced appetite after hospitalization: a phase II randomized clinical trial. Journal of the American Geriatrics Society 2005;53:970‐5. - PubMed
Ross 2001 {published data only}
-
- Ross DD, Alexander CS. Management of common symptoms in terminally ill patients: Part I. Fatigue, anorexia, cachexia, nausea and vomiting. American Family Physician 2001;64:807‐14. - PubMed
Rowland 1996 {published data only}
-
- Rowland KM, Loprinzi CL. Randomized double‐blind placebo‐controlled trial of cisplatin and etoposide plus megestrol acetate/placebo in extensive‐stage small‐cell lung cancer: a North Central Cancer Treatment Group Study. Journal of Clinical Oncology 1996;14:135‐41. - PubMed
Ruiz‐Garcia 2002 {published data only}
-
- Ruiz‐Garcia V, Juan O, Perez Hoyos S, Peiro R, Ramon N, Rosero MA, et al. Megestrol acetate: a systematic review usefulness about the weight gain in neoplastic patients with cachexia. Medicina Clinica 2002;119:166‐70. - PubMed
Sanz‐Ortiz 2004 {published data only}
-
- Sanz‐Ortiz J. Anorexia treatment in the oncological patient. Revista Clinica Espanola 2004;204:542‐4. - PubMed
Schacter 1989 {published data only}
-
- Schacter L, Rozencweig M, Canetta R, Kelley S, Nicaise C, Smaldone L. Megestrol acetate: clinical experience [Review]. Cancer Treatment Reviews 1989;16:49‐63. - PubMed
Schmoll 1992a {published data only}
-
- Schmoll E. Risks and benefits of various therapies for cancer anorexia. Oncology 1992;49 Suppl 2:43‐5. - PubMed
Simmons 2004 {published data only}
-
- Simmons SF, Walker KA, Osterweil D. The effect of megestrol acetate on oral food and fluid intake in nursing home residents: a pilot study. Journal of the American Medical Directors Association 2004;5:24‐30. - PubMed
Skarlos 1993 {published data only}
-
- Skarlos DV, Fountzilas G, Pavlidis N, Beer M, Makrantonakis P, Aravantinos G, et al. Megestrol acetate in cancer patients with anorexia and weight loss. A Hellenic Co‐operative Oncology Group (HeCOG) study. Acta Oncologica 1993;32:37‐41. - PubMed
Spaulding 1989 {published data only}
-
- Spaulding M. Recent studies of anorexia and appetite stimulation in the cancer patient. Oncology 1989;3(8 Suppl):18‐23. - PubMed
Sullivan 2007 {published data only}
-
- Sullivan DH, Roberson PK, Smith ES, Price JA, Bopp MM. Effects of muscle strength training and megestrol acetate on strength, muscle mass, and function in frail older people. Journal of the American Geriatrics Society 2007;55:20‐8. - PubMed
Tchekmedyian 1986 {published data only}
-
- Tchekmedyian NS, Tait N, Aisner J. High dose megestrol acetate in the treatment of postmenopausal women with advanced breast cancer. Seminars in Oncology 1986;13:20‐5. - PubMed
Tchekmedyian 1990 {published data only}
-
- Tchekmedyian NS, Hickman M, Siau J, Greco A, Aisner J. Treatment of cancer anorexia with megestrol acetate: impact on quality of life. Oncology 1990;4:185‐92. - PubMed
Tchekmedyian 1991 {published data only}
-
- Tchekmedyian NS, Hickman M, Heber D. Treatment of anorexia and weight loss with megestrol acetate in patients with cancer or acquired immunodeficiency syndrome. Seminars in Oncology 1991;18 Suppl 2:35‐42. - PubMed
Tchekmedyian 1993 {published data only}
-
- Tchekmedyian NS. Clinical approaches to nutritional support in cancer. Current Opinion in Oncology 1993;5:633‐8. - PubMed
Tchekmedyian 1993a {published data only}
-
- Tchekmedyian NS. Treatment of anorexia with megestrol acetate. Nutrition in Clinical Practice 1993;8:115‐8. - PubMed
Tchekmedyian 2006 {published data only}
-
- Tchekmedyian NS. Treating the anorexia/cachexia syndrome. Journal of Supportive Oncology 2006;4:506‐7. - PubMed
Thomas 2006 {published data only}
-
- Thomas DR. Guidelines for the use of orexigenic drugs in long‐term care. Nutrition in Clinical Practice 2006;21:82‐7. - PubMed
Tisdale 1993 {published data only}
-
- Tisdale MJ. Cancer cachexia. Anti‐Cancer Drugs 1993;4:115‐25. - PubMed
Tisdale 2006 {published data only}
-
- Tisdale MJ. Clinical anticachexia treatments. Nutrition in Clinical Practice 2006;21:168‐74. - PubMed
Tomiska 2003 {published data only}
-
- Tomíska M, Tomisková M, Salajka F, Adam Z, Vorlícek J. Palliative treatment of cancer anorexia with oral suspension of megestrol acetate. Neoplasma 2003;50:227‐33. - PubMed
Vigano 1994 {published data only}
-
- Vigano A, Watanabe S, Bruera E. Anorexia and cachexia in advanced cancer patients. Cancer Surveys 1994;21:99‐115. - PubMed
von Haehling 2009 {published data only}
-
- Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacology & Therapeutics 2009;121:227‐52. - PubMed
Von Roenn 1994a {published data only}
-
- Roenn JH. Randomized trials of megestrol acetate for AIDS‐associated anorexia and cachexia. Oncology 1994;51 Suppl 1:19‐24. - PubMed
Vyzula 1997 {published data only}
-
- Vyzula R. Current views on use of megestrol acetate in oncology practice. Vnitrni Lekarstvi 1997;43:250‐5. - PubMed
Westman 1999 {published data only}
-
- Westman G, Bergman B, Albertsson M, Kadar L, Gustavsson G, Thaning L, et al. Megestrol acetate in advanced, progressive, hormone‐insensitive cancer. Effects on the quality of life: a placebo‐controlled, randomised, multicentre trial. European Journal of Cancer 1999;35:586‐95. - PubMed
Yavuzsen 2005 {published data only}
-
- Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R. Systematic review of the treatment of cancer‐associated anorexia and weight loss. Journal of Clinical Oncology 2005;23:8500‐11. - PubMed
Yeh 2004 {published data only}
-
- Yeh S, Hafner A, Chang C, Levine DM, Parker TS, Schuster MW. Risk factors relating blood markers of inflammation and nutritional status to survival in cachectic geriatric patients in a randomized clinical trial. Journal of the American Geriatrics Society 2004;52:1708‐12. - PubMed
Yeh 2006 {published data only}
Yeh 2007 {published data only}
-
- Yeh SS, Lovitt S, Schuster MW. Pharmacological treatment of geriatric cachexia: evidence and safety in perspective. Journal of the American Medical Directors Association 2007;8:363‐77. - PubMed
Yeh 2010 {published data only}
-
- Yeh SS, Marandi M, Thode HC, Levine DM, Parker T, Dixon T, et al. Report of a pilot, double‐blind, placebo‐controlled study of megestrol acetate in elderly dialysis patients with cachexia. Journal of Renal Nutrition 2010; Vol. 20:52‐62. - PubMed
Zeca 1995 {published data only}
-
- Zeca E, Martini C, Venturino P, Tedeschi M, Ventafrida V, Conno F. Efficacy of megestrol acetate on anorexia in patients with advanced non hormone‐related tumors: a double‐blind placebo controlled clinical trial. European Journal of Cancer 1995;31A:S261‐2. [CN‐00519065]
Additional references
Argilés 2001
-
- Argilés JM, Meijsing SH, Pallares‐Trujillo J. Cancer cachexia. A therapeutic approach. Medical Research Review (US) 2001;21:83‐101. - PubMed
Balog 1998
-
- Balog DL, Epstein ME, Amidio‐Groton MI. HIV wasting syndrome: treatment update. Annals of Pharmacotherapy 1998;32(4):446‐58. - PubMed
Barber 1999
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54:405‐11. - PubMed
Bruera 1992
-
- Bruera E. Clinical management of anorexia and cachexia in patients with advanced cancer. Oncology 1992;49(2):35‐42. - PubMed
Campa 2005
-
- Campa A, Yang Z, Lai S, Xue L, Phillips JC, Sales S, et al. HIV‐related wasting in HIV‐infected drug users in the era of highly active antiretroviral therapy. Clinical Infectious Diseases 2005;41:1179‐85. - PubMed
Deeks 2008
-
- Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from: www.cochrane‐handbook.org.
Fearon 2011
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncology 2011;12:489‐95. - PubMed
Fick 2012
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Chichester, UK: Wiley‐Blackwell, 2011:649.
Jadad 1996
-
- Jadad A, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports on randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;170:1‐12. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. - PubMed
Laird 1990
-
- Laird NM, Wang F. Estimating rates of change in randomized clinical trials. Controlled Clinical Trials 1990;11:405‐19. - PubMed
Laupacis 1988
-
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine 1988;318:1728‐33. - PubMed
Loprinzi 1990a
-
- Loprinzi C, Ellison NM, Goldberg RM, Michalak JC, Burch PA. Alleviation of cancer anorexia and cachexia: studies of the Mayo Clinic and the North Central Cancer Treatment Group. Seminars in Oncology 1990;17(6 Suppl 9):8‐12. - PubMed
Loprinzi 1999
-
- Loprinzi CL, Kugler JW, Sloan JA. Randomized comparison of megestrol acetate versus dexamethasone versus fluoxymesterone for the treatment of cancer anorexia/cachexia. Journal of Clinical Oncology 1999;17:3299‐306. - PubMed
Mangili 2006
-
- Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clinical Infectious Diseases 2006;42:836‐42. - PubMed
Nelson 1994
-
- Nelson KA, Walsh D, Sheehan FA. The cancer anorexia‐cachexia syndrome. Journal of Clinical Oncology 1994;12:213‐25. - PubMed
Nüesch 2010
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schulz 1993
-
- Schulz KF, Altman DG. Statistical Methods for Data Synthesis. Cochrane Workshop Report. Oxford: UK Cochrane Centre, 1993.
Splinter 1992
-
- Splinter TA. Cachexia and cancer: a clinician's view. Annals of Oncology 1992;3(3):25‐7. - PubMed
Tang 2005
-
- Tang AM, Jacobson DL, Spiegelman D, Knox TA, Wanke CA. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV‐infected patients, 1995 to 2003. Journal of Acquired Immune Deficiency Syndromes 2005;40:70‐6. - PubMed
Thompson 1952
-
- Thompson CM, Rodgers RL. Analysis of the autopsy records of 157 cases of carcinoma of the pancreas with particular reference to the incidence of thromboembolism. American Journal of the Medical Sciences 1952;223:469‐76. - PubMed
Thompson 1999
-
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18:2693‐708. - PubMed
Tisdale 2009
-
- Tisdale M. Mechanisms of cancer cachexia. Physiological Reviews 209;89:381‐410. - PubMed
Verso 2003
-
- Verso M, Agnelli G. Venous thromboembolism associated with long‐term use of central venous catheters in cancer patients. Journal of Clinical Oncology 2003;21:3665‐75. - PubMed
Von Roenn 1996
-
- Roenn JH, Knopf K. Anorexia/cachexia in patients with HIV: lessons for the oncologist. Oncology 1996;10(7):1049‐56. - PubMed
Wigmore 1997
-
- Wigmore SJ, Fearon KCH, Maingay JP, Ross JA. Down‐regulation of the acute‐phase response in patients with pancreatic cancer cachexia receiving oral eicosapentaenoic acid is mediated via suppression of interleukin‐6. Clinical Science 1997;92:215‐21. - PubMed
Wolf 2006
-
- Wolf I, Sadetzki S, Kanety H, Kundel Y, Pariente C, Epstein N, et al. Adiponectin, ghrelin and leptin in cancer cachexia in breast and colon cancer patients. Cancer 2006;106:966‐73. - PubMed
Yusuf 1991
-
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266:93‐8. - PubMed
References to other published versions of this review
Berenstein G 2005
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

