Topical treatments for chronic plaque psoriasis
- PMID: 23543539
- PMCID: PMC11227123
- DOI: 10.1002/14651858.CD005028.pub3
Topical treatments for chronic plaque psoriasis
Abstract
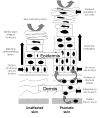
Background: Chronic plaque psoriasis is the most common type of psoriasis, and it is characterised by redness, thickness, and scaling. First-line management of chronic plaque psoriasis is with topical treatments, including vitamin D analogues, topical corticosteroids, tar-based preparations, dithranol, salicylic acid, and topical retinoids.
Objectives: To compare the effectiveness, tolerability, and safety of topical treatments for chronic plaque psoriasis, relative to placebo, and to similarly compare vitamin D analogues (used alone or in combination) with other topical treatments.
Search methods: We updated our searches of the following databases to February 2011: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2011, Issue 2), MEDLINE (from 1948), EMBASE (from 1980), Science Citation Index (from 2008), Conference Proceedings Citation Index - Science (from 2008), BIOSIS (from 1993), Dissertation Abstracts via DialogClassic (all publication years), and Inside Conferences (all publication years).We identified ongoing and unpublished studies from the UK Clinical Research Network Study Portfolio and the metaRegister of Controlled Trials. We checked the bibliographies of published studies and reviews for further references to relevant trials, and we contacted trialists and companies for information about newly published studies.A separate search for adverse effects was undertaken in February 2011 using MEDLINE and EMBASE (from 2005).Final update searches for both RCTs and adverse effects were undertaken in August 2012. Although it has not been possible to incorporate RCTs and adverse effects studies identified through these final searches within this review, we will incorporate these into the next update.
Selection criteria: Randomised trials comparing active topical treatments against placebo or against vitamin D analogues (used alone or in combination) in people with chronic plaque psoriasis.
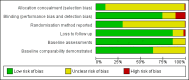
Data collection and analysis: One author extracted study data and assessed study quality. A second author checked these data. We routinely contacted trialists and companies for missing data. We also extracted data on withdrawals and on local and systemic adverse events. We defined long-term trials as those with a duration of at least 24 weeks.
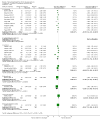
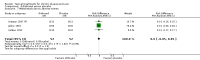
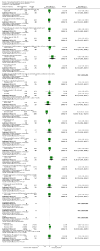
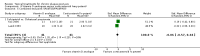
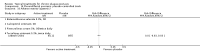
Main results: This update added 48 trials and provided evidence on 7 new active treatments. In total, the review included 177 randomised controlled trials, with 34,808 participants, including 26 trials of scalp psoriasis and 6 trials of inverse psoriasis, facial psoriasis, or both. The number of included studies counted by Review Manager (RevMan) is higher than these figures (190) because we entered each study reporting a placebo and an active comparison into the 'Characteristics of included studies' table as 2 studies.When used on the body, most vitamin D analogues were significantly more effective than placebo, with the standardised mean difference (SMD) ranging from -0.67 (95% CI -1.04 to -0.30; 1 study, 119 participants) for twice-daily becocalcidiol to SMD -1.66 (95% CI -2.66 to -0.67; 1 study, 11 participants) for once-daily paricalcitol. On a 6-point global improvement scale, these effects translate into 0.8 and 1.9 points, respectively. Most corticosteroids also performed better than placebo; potent corticosteroids (SMD -0.89; 95% CI -1.06 to -0.72; I² statistic = 65.1%; 14 studies, 2011 participants) had smaller benefits than very potent corticosteroids (SMD -1.56; 95% CI -1.87 to -1.26); I² statistic = 81.7%; 10 studies, 1264 participants). On a 6-point improvement scale, these benefits equate to 1.0 and 1.8 points, respectively. Dithranol, combined treatment with vitamin D/corticosteroid, and tazarotene all performed significantly better than placebo.Head-to-head comparisons of vitamin D for psoriasis of the body against potent or very potent corticosteroids had mixed findings. For both body and scalp psoriasis, combined treatment with vitamin D and corticosteroid performed significantly better than vitamin D alone or corticosteroid alone. Vitamin D generally performed better than coal tar, but findings relative to dithranol were mixed. When applied to psoriasis of the scalp, vitamin D was significantly less effective than both potent corticosteroids and very potent corticosteroids. Indirect evidence from placebo-controlled trials supported these findings.For both body and scalp psoriasis, potent corticosteroids were less likely than vitamin D to cause local adverse events, such as burning or irritation. Combined treatment with vitamin D/corticosteroid on either the body or the scalp was tolerated as well as potent corticosteroids, and significantly better than vitamin D alone. Only 25 trials assessed clinical cutaneous dermal atrophy; few cases were detected, but trials reported insufficient information to determine whether assessment methods were robust. Clinical measurements of dermal atrophy are insensitive and detect only the most severe cases. No comparison of topical agents found a significant difference in systemic adverse effects.
Authors' conclusions: Corticosteroids perform at least as well as vitamin D analogues, and they are associated with a lower incidence of local adverse events. However, for people with chronic plaque psoriasis receiving long-term treatment with corticosteroids, there remains a lack of evidence about the risk of skin dermal atrophy. Further research is required to inform long-term maintenance treatment and provide appropriate safety data.
Conflict of interest statement
Mike Cork: "I gave a lecture for Leo Pharmaceuticals about psoriasis and atopic eczema in 2012."
Anne R Mason: none declared
Gordon Dooley: none declared
James Mason: none declared
Helen Hancock: none declared
The clinical referee for this review, Dr Phyllis Spuls, stated the following potential conflict of interest on her comments form: "I have participated in an advisory board of LEO Pharma to give guidance to general practitioners regarding what to do if patients used calcipotriol, which has been removed from the market. I am involved as a Principal Investigator in many clinical trials with systemic agents for psoriasis and now in one for the improvement of adherence to Dovobet."
Figures


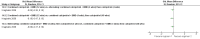




























































































































































Update of
-
Topical treatments for chronic plaque psoriasis.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD005028. doi: 10.1002/14651858.CD005028.pub2. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2013 Mar 28;(3):CD005028. doi: 10.1002/14651858.CD005028.pub3. PMID: 19370616 Updated.
References
References to studies included in this review
Agrup 1981 {published data only}
-
- Agrup G, Bjornberg A, Elmros T, Groth O, Hannuksela M, Lassus A, et al. Clinical Trial of a Potent Non‐Halogenated Topical Steroid, Budesonide. Acta Dermato Venereologica 1981;61(2):180‐2. [PUBMED: 6165205] - PubMed
Alora‐Palli 2010 {published and unpublished data}
-
- Alora‐Palli MB, Kimball AB, Cott A. A new topically applied liquor carbonis distillate (coal tar) solution helps reduce regression of plaque psoriasis after 12 weeks of treatment: A 6‐week comparison to calcipotriol cream. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB174.
-
- Alora‐Palli Maria B, Perkins Alexis C, Cott Alicia, Kimball Alexandra B. Efficacy and tolerability of a cosmetically acceptable coal tar solution in the treatment of moderate plaque psoriasis: a controlled comparison with calcipotriene (calcipotriol) cream. American Journal of Clinical Dermatology 2010;11(4):275‐83. [PUBMED: 20513160] - PubMed
-
- Brouda I, Edison B, Cott A, Green BA. Tolerability and cosmetic acceptability of liquor carbonis distillate (coal tar) solution 15% as topical therapy for plaque psoriasis. Cutis 2010;85(4):214‐20. - PubMed
Austad 1998 {published and unpublished data}
-
- Austad J, Bjerke JR, Gjertsen BT, Helland S, Livden JK, Morken T, et al. Clobetasol Propionate Followed by Calcipotriol Is Superior to Calcipotriol Alone in Topical Treatment of Psoriasis. Journal of the European Academy of Dermatology & Venereology 1998;11(1):19‐24. [PUBMED: 9731961] - PubMed
Baiocchi 1997 {published data only}
-
- Baiocchi R, Bertani E, Biggio P, Calandra P, Marchi R, Franchi A, et al. Controlled Trial of the Efficacy and Safety of Calcipotriol Ointment Applied Once or Twice a Day in Psoriasis Vulgaris Studio [Controllato Per Emiparti Sull'efficacia E La Tollerabilita Di Calcipotriolo Unguento 1 Applicazione/Die Versus 2 Applicazioni/Die, Nella Psoriasi Volgare]. Giornale Italiano di Dermatologia e Venereologia 1997;132(2):139‐47.
Barker 1999 (H) {published and unpublished data}
-
- Barker N, Ashton RE, Marks R, Harris RI, Berth‐Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo‐controlled, double‐blind, dose‐finding study with active comparator. British Journal Of Dermatology 1999;141(2):274‐8. [MEDLINE: ] - PubMed
Barker 1999 (P) {published and unpublished data}
-
- Barker N, Ashton RE, Marks R, Harris RI, Berth‐Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo‐controlled, double‐blind, dose‐finding study with active comparator. British Journal Of Dermatology 1999;141(2):274‐8. [PUBMED: 10468799] - PubMed
-
- Berth‐Jones J. Maxacalcitol ‐ a new, potent and safe vitamin D analogue for treatment of psoriasis. British Journal Of Dermatology 1999;141:992. - PubMed
Barrett 2005 {published and unpublished data}
-
- Barrett C, Lowson D, Blades K J, investigators Mcs Int. Limited benefit of combined use of tar‐based shampoo with 50 microg/ml calcipotriol solution in scalp psoriasis. Journal of Dermatological Treatment 2005;16(3):175. [PUBMED: 16211775] - PubMed
Bernhard 1991 (1) {published data only}
-
- Bernhard J, Whitmore C, Guzzo C, Kantor I, Kalb RE, Ellis C, et al. Evaluation of Halobetasol Propionate Ointment in the Treatment of Plaque Psoriasis: Report on Two Double‐Blind, Vehicle‐Controlled Studies. Journal of the American Academy of Dermatology 1991;25(6 Pt 2):1170‐4. [PUBMED: 1757612] - PubMed
Bernhard 1991(2) {published data only}
-
- Bernhard J, Whitmore C, Guzzo C, Kantor I, Kalb RE, Ellis C, et al. Evaluation of Halobetasol Propionate Ointment in the Treatment of Plaque Psoriasis: Report on Two Double‐Blind, Vehicle‐Controlled Studies. Journal of the American Academy of Dermatology 1991;25(6 Pt 2):1170‐4. [PUBMED: 1757612] - PubMed
Bernstein 2006 {published data only}
-
- Bernstein S, Donsky H, Gulliver W, Hamilton D, Nobel S, Norman R. Treatment of mild to moderate psoriasis with Relieva, a Mahonia aquifolium extract‐‐a double‐blind, placebo‐controlled study. American Journal of Therapeutics 2006; Vol. 13, issue 2:121‐6. [PUBMED: 16645428] - PubMed
Berth Jones 1992b {published data only}
-
- Berth Jones J, Chu AC, Dodd WA, Ganpule M, Griffiths WA, Haydey RP, et al. A Multicentre, Parallel‐Group Comparison of Calcipotriol Ointment and Short‐Contact Dithranol Therapy in Chronic Plaque Psoriasis. British Journal Of Dermatology 1992;127(3):266‐71. [PUBMED: 1390171] - PubMed
Beutner 2006 {published data only}
-
- Beutner K, Chakrabarty A. An intra‐individual safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. [Abstract P2858.] American Academy of Dermatology 64th Annual Meeting 3‐7 March 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB212. - PubMed
-
- Beutner K, Chakrabarty A, Lemke S, Yu K. An intra‐individual randomized safety and efficacy comparison of clobetasol propionate 0.05% spray and its vehicle in the treatment of plaque psoriasis. Journal of Drugs in Dermatology 2006;5(4):357‐60. [PUBMED: 16673804] - PubMed
Bourke 1993b {published data only}
-
- Bourke JF, Berth‐Jones J, Hutchinson PE. Occlusion enhances the efficacy of topical calcipotriol in the treatment of psoriasis vulgaris. Clinical & Experimental Dermatology 1993;18(6):504‐6. [PUBMED: 8252786] - PubMed
Bourke 1997 {published and unpublished data}
-
- Bourke JF, Featherstone S, Iqbal SJ, Hutchinson PE. A Double‐Blind Comparison of Topical Calcitriol (3 mcg/g) and Calcipotriol (50 mcg/g) in the Treatment of Chronic Plaque Psoriasis Vulgaris. British Journal Of Dermatology 1995;133(Suppl 45):17.
-
- Bourke JF, Iqbal SJ, Hutchinson PE. A Randomized Double‐Blind Comparison of the Effects on Systemic Calcium Homeostasis of Topical Calcipotriol (3 Mu G/G) and Calcipotriol (50 U M/G) in the Treatment of Chronic Plaque Psoriasis Vulgaris. Acta Dermato Venereologica 1997;77(3):228‐30. [PUBMED: 9188878] - PubMed
Brown 2005 {published and unpublished data}
-
- Brown AC, Koett J, Johnson DW, Semaskvich NM, Holck P, Lally D, et al. Effectiveness of kukui nut oil as a topical treatment for psoriasis. International Journal of Dermatology 2005; Vol. 44, issue 8:684‐7. [PUBMED: 16101874] - PubMed
Bruce 1994 {published and unpublished data}
-
- Bruce S, Epinette WW, Funicella T, Ison A, Jones EL, Loss RJ, et al. Comparative Study of Calcipotriene (Mc 903) Ointment and Fluocinonide Ointment in the Treatment of Psoriasis. Journal of the American Academy of Dermatology 1994;31(5 Pt 1):755‐9. [PUBMED: 7929921] - PubMed
-
- Siskin SB. Efficacy and Safety of Calcipotriol Ointment Compared to Fluocinonide. 2nd International Symposium on Calcipotriol. Monaco, 1993.
Buckley 1978 {published data only}
-
- Buckley DB. A Double‐Blind Comparison of 0.1% Dithranol in a 17% Urea Base ("Psoradrate") and Base Alone in the Treatment of Active Chronic Psoriasis. Current Medical Research & Opinion 1978;5(6):489‐94. [PUBMED: 350501 ] - PubMed
Buckley 2008 {published and unpublished data}
-
- Buckley C, Hoffmann V. Calcipotriol plus betamethasone dipropionate gel is effective and safe in the treatment of scalp psoriasis (a phase II study). [Abstract P06.57.] 14th Congress of the European Academy of Dermatology and Venereology, 12‐15th October 2005, London UK. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):175.
-
- Buckley C, Hoffmann V, Shapiro J, Saari S, Cambazard F, Milsgaard M. Calcipotriol plus betamethasone dipropionate scalp formulation is effective and well tolerated in the treatment of scalp psoriasis: a phase II study. Dermatology 2008; Vol. 217, issue 2:107‐13. [PUBMED: 18463448] - PubMed
Camarasa 2003 {published data only}
-
- Camarasa JM, Ortonne JP, Dubertret L. Calcitriol shows greater persistence of treatment effect than betamethasone dipropionate in topical psoriasis therapy. Journal of Dermatological Treatment 2003;14(1):8‐13. [PUBMED: 12745849] - PubMed
Cheesbrough 1992 {published data only}
-
- Cheesbrough MJ. Treatment of psoriasis with 30% Dead Sea salt lotion. Journal Of Dermatological Treatment 1992;3(4):201‐203. [EMBASE: 1993020259]
Christensen 1999 {published data only}
-
- Christensen OB, Mork NJ, Ashton R, Daniel F, Anehus S. Comparison of a treatment phase and a follow‐up phase of short‐contact dithranol and calcipotriol in outpatients with chronic plaque psoriasis. Journal Of Dermatological Treatment 1999;10(4):261‐5. [EMBASE: 2000044219]
Cook‐Bolden 2010 {published and unpublished data}
-
- Cook‐Bolden FE, Goffe BS, Hudson CP, Sofen HL. Efficacy and safety results from a randomized, double‐blind, vehicle‐controlled study of clobetasol propionate spray for the treatment of moderate to severe plaque psoriasis of the scalp [Abstract]. Conference: 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL United States. Conference Start: 20100305 Conference End: 20100309. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB140. [EMBASE: 70142994; NCT00881868]
-
- Cook‐Bolden FE, Goffe BS, Hudson CP, Sofen HL. Patient satisfaction with clobetasol propionate spray, 0.05% for the management of moderate to severe plaque psoriasis of the scalp [Abstract]. Conference: 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States. Conference Start: 20120316 Conference End: 20120320. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB197. [EMBASE: 70704639]
-
- Cook‐Bolden FE, Goffe BS, Sofen HL, Colon LE, Gottschalk RW. Successful treatment of moderate to severe plaque psoriasis of the scalp with clobetasol propionate spray, 0.05% [Abstract]. Conference: 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB157. [EMBASE: 70331905]
-
- Cook‐Bolden FE, Goffe BS, Sofen HL, Hudson CP, Colon LE, Gottschalk RW, et al. Patient satisfaction with clobetasol propionate spray, 0.05% for the management of moderate to severe plaque psoriasis of the scalp [Abstract]. Conference: 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States. Conference Start: 20120219 Conference End: 20120224.. Seminars in Cutaneous Medicine & Surgery 2012;31(1):A9‐A10. [EMBASE: 70706927]
-
- Jarratt M, Sofen HL, Caveney S. The effects of clobetasol propionate spray, 0.05% vs. vehicle spray on the signs of plaque psoriasis on the body and scalp [Abstract]. Conference: 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States. Conference Start: 20120219 Conference End: 20120224. Seminars in Cutaneous Medicine & Surgery 2012;31(1):A11. [EMBASE: 70706930]
Crosti 1997 {published data only}
-
- Crosti C, Finzi AF, Mian E, Scarpa C. Calcipotriol in psoriasis vulgaris: a controlled trial comparing betamethasone dipropionate + salicylic acid. International Journal Of Dermatology 1997;36(7):537‐9. [PUBMED: 9268756] - PubMed
Cunliffe 1992 {published data only}
-
- Cunliffe WJ, Berth Jones J, Claudy A, Fairiss GM, Goldin D, Gratton D, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17‐valerate ointment in patients with psoriasis vulgaris. Journal of the American Academy of Dermatology 1992;26(5 Pt 1):736‐43. [MEDLINE: ] - PubMed
Decroix 2004 {published data only}
-
- Decroix J, Pres H, Tsankov N, Poncet M, Arsonnaud S. Clobetasol propionate lotion in the treatment of moderate to severe plaque‐type psoriasis. Cutis 2004;74(3):201‐6. [PUBMED: 15499763] - PubMed
De Simone 1993 {published data only}
-
- Simone C, Guerriero C, Morini P, Venier A. Treatment of Psoriasis with Topical Calcipotriol [Il Calcipotriol Nella Terapia Locale Della Psoriasi]. Giornale Italiano di Dermatologia e Venereologia 1993;128(10):Ic‐Ciii.
Douglas 2002 {published and unpublished data}
-
- Douglas WS, Poulin Y, Decroix J, Ortonne JP, Mrowietz U, Gulliver W, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Dermato Venereologica 2002;82(2):131‐5. [PUBMED: 12125943 ] - PubMed
Dubertret 1992 {published and unpublished data}
-
- Dubertret L, Wallach D, Souteyrand P, Perussel M, Kalis B, Meynadier J, et al. Efficacy and Safety of Calcipotriol (Mc 903) Ointment in Psoriasis Vulgaris. A Randomized, Double‐Blind, Right/Left Comparative, Vehicle‐Controlled Study. Journal of the American Academy of Dermatology 1992;27(6 Pt 1):983‐8. [PUBMED: 1479106] - PubMed
Durakovic 2001 {published data only}
-
- Durakovic C, Malabanan A, Holick MF. Rationale for use and clinical responsiveness of hexafluoro‐1,25‐dihydroxyvitamin D3 for the treatment of plaque psoriasis: a pilot study. British Journal Of Dermatology 2001;144(3):500‐6. [PUBMED: 11260006] - PubMed
Durakovic 2004 {published data only}
-
- Durakovic C, Ray S, Holick MF. Topical paricalcitol (19‐nor‐1 alpha,25‐dihydroxyvitamin D2) is a novel, safe and effective treatment for plaque psoriasis: a pilot study. British Journal of Dermatology 2004;151(1):190‐5. [PUBMED: 15270890] - PubMed
Duweb 2000 {published data only}
-
- Duweb GA, Abuzariba O, Rahim M, al‐Taweel M, Abdulla SA. Scalp psoriasis: topical calcipotriol 50 micrograms/g/ml solution vs. betamethasone valerate 1% lotion. International Journal of Clinical Pharmacology Research 2000;20(3‐4):65‐8. [PUBMED: 11314240 ] - PubMed
Elie 1983 {published data only}
-
- Elie R, Durocher LP, Kavalec EC. Effect of Salicylic Acid on the Activity of Betamethasone‐17,21‐Dipropionate in the Treatment of Erythematous Squamous Dermatoses. Journal of International Medical Research 1983;11(2):108‐12. [PUBMED: 6852357 ] - PubMed
Ellis 1988 {published data only}
-
- Ellis CN, Horwitz SN, Menter A. Amcinonide Lotion 0.1% in the Treatment of Patients with Psoriasis of the Scalp. Current Therapeutic Research ‐ Clinical & Experimental 1988;44(2):315‐24. [EMBASE: 1989002095]
Escobar 1992 {published data only}
-
- Escobar SO, Achenbach R, Iannantuono R, Torem V. Topical fish oil in psoriasis‐‐a controlled and blind study. Clinical & Experimental Dermatology 1992;17(3):159‐62. [PUBMED: 1451289 ] - PubMed
Farkas 1999 {published and unpublished data}
-
- Farkas B, Dobozy A. Comparison of Tacalcitol with Dithranol. 3rd European Hermal Symposium. Hamburg, 1995.
-
- Farkas B, Dobozy A, Horvath A, Hunyadi J, Schneider I. Comparison of tacalcitol ointment with short‐contact dithranol therapy in the treatment of psoriasis vulgaris: a randomized multicentre, open prospective study on efficacy and safety. Journal of Dermatological Treatment 1999;10(2):93‐9. [EMBASE: 1999259658]
Feldman 2010 (1) {published data only (unpublished sought but not used)}
-
- Feldman S, Wyres M, Markowitz O, Matheson R, Bruce S. Calcipotriene foam for the treatment of plaque‐type psoriasis: Results of two phase III studies. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB138. [NCT00689481]
-
- Feldman SR, Matheson R, Bruce S, Grande K, Markowitz O, Kempers S, et al. Efficacy and safety of calcipotriene 0.005% foam for the treatment of plaque‐type psoriasis: Results of two multicenter, randomized, double‐blind, vehicle‐controlled, phase III clinical trials. American Journal of Clinical Dermatology 2012;13(4):261‐71. [EMBASE: 2012323159] - PubMed
-
- NCT00689481. Study to Compare U0267 Against Vehicle in Subjects With Plaque‐type Psoriasis Two of Two Phase 3 Studies. clinicaltrials.gov/ct2/results?term=NCT00689481 (accessed 2010).
Feldman 2010 (2) {published data only (unpublished sought but not used)}
-
- Feldman S R, Matheson R, Bruce S, Grande K, Markowitz O, Kempers S, et al. Efficacy and safety of calcipotriene 0.005% foam for the treatment of plaque‐type psoriasis: Results of two multicenter, randomized, double‐blind, vehicle‐controlled, phase III clinical trials. AMERICAN JOURNAL OF CLINICAL DERMATOLOGY 2012;13(4):261‐71. - PubMed
-
- Feldman S, Wyres M, Markowitz O, Matheson R, Bruce S. Calcipotriene foam for the treatment of plaque‐type psoriasis: Results of two phase III studies. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB138. [NCT00689481]
-
- NCT00688519. Study to Compare U0267 Against Vehicle in Subjects With Plaque‐type Psoriasis One of Two Phase 3 Studies. clinicaltrials.gov/ct2/results?term=NCT00688519 (accessed 2010).
Fleming 2010 (H) {published and unpublished data}
-
- Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double‐blind, exploratory study. European Journal of Dermatology 2010;20(4):465‐71. [PUBMED: 20413372] - PubMed
-
- Fleming C, Ganslandt C, Leese GP. Short‐ and long‐term safety assessment of a two‐compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex/Daivobet/Dovobet ointment): hypothalamic‐pituitary‐adrenal axis function in patients with psoriasis vulgaris. Journal of Drugs in Dermatology 2010;9(8):969‐74. [PUBMED: 20684147] - PubMed
Fleming 2010 (P) {published and unpublished data}
-
- Fleming C, Ganslandt C, Guenther L, Johannesson A, Buckley C, Simon JC, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double‐blind, exploratory study. European Journal of Dermatology 2010;20(4):465‐71. [PUBMED: 20413372] - PubMed
-
- Fleming C, Ganslandt C, Leese GP. Short‐ and long‐term safety assessment of a two‐compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex/Daivobet/Dovobet ointment): hypothalamic‐pituitary‐adrenal axis function in patients with psoriasis vulgaris. Journal of Drugs in Dermatology 2010;9(8):969‐74. [PUBMED: 20684147] - PubMed
Franz 1999 {published data only}
-
- Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12%: a novel vehicle with enhanced delivery and efficacy. International Journal Of Dermatology 1999;38(8):628‐32. [PUBMED: 10487457 ] - PubMed
Franz 2000 {published data only}
-
- Franz TJ, Parsell DA, Myers JA, Hannigan JF. Clobetasol propionate foam 0.05%: a novel vehicle with enhanced delivery. International Journal Of Dermatology 2000;39(7):535‐8. [PUBMED: 10940121 ] - PubMed
Geilen 2000 {published data only}
-
- Geilen CC, Mrowietz U. Lack of efficacy of topical mycophenolic acid in psoriasis vulgaris. Journal of the American Academy of Dermatology 2000;42(5 Pt 1):837‐40. [PUBMED: 10775867 ] - PubMed
Gottlieb 2003 {published data only}
-
- Gottlieb AB, Ford RO, Spellman MC. The efficacy and tolerability of clobetasol propionate foam 0.05% in the treatment of mild to moderate plaque‐type psoriasis of nonscalp regions. Journal of Cutaneous Medicine & Surgery 2003;7(3):185‐92. [PUBMED: 12704534 ] - PubMed
Grattan 1997 (H) {published data only}
-
- Grattan CEH, Hallam F, Whitefield M. A New Aqueous Dithranol Gel for Psoriasis: Comparison with Placebo and Calcipotriol Ointment. Journal Of Dermatological Treatment 1997;8(1):11‐5. [EMBASE: 1997109958]
Grattan 1997 (P) {published data only}
-
- Grattan CEH, Hallam F, Whitefield M. A New Aqueous Dithranol Gel for Psoriasis: Comparison with Placebo and Calcipotriol Ointment. Journal Of Dermatological Treatment 1997;8(1):11‐5. [EMBASE: 1997109958]
Green 1994 {published and unpublished data}
-
- Green C, Ganpule M, Harris D, Kavanagh G, Kennedy C, Mallett R, et al. Comparative Effects of Calcipotriol (Mc903) Solution and Placebo (Vehicle of Mc903) in the Treatment of Psoriasis of the Scalp. British Journal Of Dermatology 1994;130(4):483‐7. [PUBMED: 8186114 ] - PubMed
Greenspan 1993 {published and unpublished data}
-
- Greenspan A, Herndon JJ, Baker MD, Cheney T. Controlled Evaluation of 0.05% Desonide Lotion and Desonide Cream in Psoriasis. Current Therapeutic Research ‐ Clinical & Experimental 1993;53(6):614‐20. [EMBASE: 1993261649]
Gribetz 2004 {published and unpublished data}
-
- Gribetz C, Ling M, Lebwohl M, Pariser D, Draelos Z, Gottlieb AB, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: A double‐blind, randomized study. Journal of the American Academy of Dermatology 2004;51(5):731‐8. [PUBMED: 15523351] - PubMed
Guenther 2000 {published data only}
-
- Guenther LC, Poulin YP, Pariser DM. A comparison of tazarotene 0.1% gel once daily plus mometasone furoate 0.1% cream once daily versus calcipotriene 0.005% ointment twice daily in the treatment of plaque psoriasis. Clinical Therapeutics 2000;22(10):1225‐38. [PUBMED: 11110233] - PubMed
Guenther 2002 (H) {published and unpublished data}
-
- Guenther L, Kerkhof PCM, Kragballe K, Chu AC, Tegner E, Garcia‐Diez A, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double‐blind, vehicle‐controlled clinical trial. British Journal Of Dermatology 2002;147(2):316‐23. [PUBMED: 12174105] - PubMed
-
- Kerkhof PCM. Once daily vs. twice daily applications of topical treatments in psoriasis [15]. British Journal of Dermatology 2005; Vol. 153, issue 6:1245. - PubMed
-
- Kerkhof PC. The impact of a two‐compound product containing calcipotriol and betamethasone dipropionate (Daivobet/ Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. British Journal of Dermatology 2004;151(3):663‐8. [PUBMED: 15377355] - PubMed
-
- Kerkhof PCM. A fixed combination of calcipotriol/betamethasone dipropionate improves quality of life in patients with psoriasis vulgaris. 11th Congress of the European Academy of Dermatology and Venereology; 2002 Oct 2‐6; Prague. 2002:27‐2.
Guenther 2002 (P) {published and unpublished data}
-
- Guenther L, Kerkhof PCM, Kragballe K, Chu AC, Tegner E, Garcia‐Diez A, et al. Efficacy and safety of a new combination of calcipotriol and betamethasone dipropionate (once or twice daily) compared to calcipotriol (twice daily) in the treatment of psoriasis vulgaris: a randomized, double‐blind, vehicle‐controlled clinical trial. British Journal Of Dermatology 2002;147(2):316‐23. [PUBMED: 12174105] - PubMed
-
- Kerkhof PCM. Once daily vs. twice daily applications of topical treatments in psoriasis [15]. British Journal of Dermatology 2005; Vol. 153, issue 6:1245. - PubMed
-
- Kerkhof PC. The impact of a two‐compound product containing calcipotriol and betamethasone dipropionate (Daivobet/ Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. British Journal of Dermatology 2004;151(3):663‐8. [MEDLINE: ] - PubMed
-
- Kerkhof PCM. A fixed combination of calcipotriol/betamethasone dipropionate improves quality of life in patients with psoriasis vulgaris. 11th Congress of the European Academy of Dermatology and Venereology; 2002 Oct 2‐6; Prague. 2002:P27‐2.
Han 2001 {published data only}
-
- Han GW, Yu BT, Li H, Zhu XJ, Wang BX, Li GM, et al. A randomized controlled multicenter clinical trial on tazarotene gel versus calcipotriol ointment in the treatment of plaque psoriasis vulgaris. Chinese Journal of Clinical Pharmacology 2001;17(6):419‐22.
Harrington 1996a {published data only}
-
- Harrington CI, Goldin D, Lovell CR, Kerkhof P, Nieboer C, Austad J, et al. Comparative Effects of Two Different Calcipotriol (Mc 903) Cream Formulations Versus Placebo in Psoriasis Vulgaris. A Randomised, Double‐Blind, Placebo‐Controlled, Parallel Group Multi‐Centre Study 1. Journal of the European Academy of Dermatology & Venereology 1996;6(2):152‐8. [EMBASE: 1996096735]
Helfrich 2007 {published and unpublished data}
-
- Helfrich YR, Kang S, Hamilton TA, Voorhees JJ. Topical becocalcidiol for the treatment of psoriasis vulgaris: a randomized, placebo‐controlled, double‐blind, multicentre study. British Journal of Dermatology 2007; Vol. 157, issue 2:369‐74. - PubMed
Henneicke‐v. Z. 1993 {published data only}
-
- Henneicke‐von Zepelin HH, Mrowietz U, Farber L, Bruck‐Borchers K, Schober C, Huber J, et al. Highly purified omega‐3‐polyunsaturated fatty acids for topical treatment of psoriasis. Results of a double‐blind, placebo‐controlled multicentre study. British Journal Of Dermatology 1993;129(6):713‐7. [PUBMED: 8286257] - PubMed
Highton 1995 {published data only}
-
- Highton A, Quell J, Breneman D, Cullen S, Goffe B, Griffiths C, et al. Calcipotriene ointment 0.005% for psoriasis: A safety and efficacy study. Journal of the American Academy of Dermatology 1995;32(1):67‐72. [PUBMED: 7822519] - PubMed
Hindsén 2006 (H) {published and unpublished data}
-
- Hindsén M, Traulsen J. Calcipotriol ointment under occlusion gives a fast onset of action. Journal of the European Academy of Dermatology & Venereology 2006;20(6):764‐5. [PUBMED: 16836527] - PubMed
Hindsén 2006 (P) {published and unpublished data}
-
- Hindsén M, Traulsen J. Calcipotriol ointment under occlusion gives a fast onset of action. Journal of the European Academy of Dermatology & Venereology 2006;20(6):764‐5. [PUBMED: 16836527] - PubMed
Holick 2003 {published data only}
-
- Holick MF, Chimeh FN, Ray S. Topical PTH (1‐34) is a novel, safe and effective treatment for psoriasis: a randomized self‐controlled trial and an open trial. British Journal of Dermatology 2003;149(2):370‐6. [PUBMED: 12932245] - PubMed
Huang 2009 {published data only}
-
- Huang L, Ma L, Huang Q, Yang QP, Zheng ZZ, Zhu XJ, et al. Calcipotriol betamethasone ointment in the treatment of psoriasis vulgaris: a randomized, double‐blind, active‐controlled, parallel group study. Zhonghua Pifuke Zazhi (Chinese Journal of Dermatology) 2009;42(10):691‐4. [NCT00248456]
Hutchinson 2000 {published data only}
-
- Hutchinson PE, Marks R, White J. The efficacy, safety and tolerance of calcitriol 3 microg/g ointment in the treatment of plaque psoriasis: a comparison with short‐contact dithranol. Dermatology 2000;201(2):139‐45. [PUBMED: 11053917] - PubMed
Jarratt 2004 {published data only}
-
- Jarratt M, Breneman D, Gottlieb AB, Poulin Y, Liu Y, Foley V. Clobetasol propionate shampoo 0.05%: a new option to treat patients with moderate to severe scalp psoriasis. Journal of Drugs in Dermatology 2004;3(4):367‐73. [PUBMED: 15303780] - PubMed
Jarratt 2006 {published and unpublished data}
-
- Jarratt M, Sofen HL, Caveney S. The effects of clobetasol propionate spray, 0.05% vs. vehicle spray on the signs of plaque psoriasis on the body and scalp [Abstract]. Conference: 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States. Conference Start: 20120219 Conference End: 20120224. Seminars in Cutaneous Medicine & Surgery 2012;31(1):A11. [EMBASE: 70706930]
-
- Jarratt MT, Clark SD, Savin RC, Swinyer LJ, Safley CF, Brodell RT, et al. Evaluation of the efficacy and safety of clobetasol propionate spray in the treatment of plaque‐type psoriasis. Cutis 2006; Vol. 78, issue 5:348‐54. [PUBMED: 17186795] - PubMed
Jekler 1992 {published data only}
-
- Jekler J, Swanbeck G. One‐Minute Dithranol Therapy in Psoriasis: A Placebo‐Controlled Paired Comparative Study. Acta Dermato Venereologica 1992;72(6):449‐50. [PUBMED: 1362841] - PubMed
Jemec 2008 (H) {published and unpublished data}
-
- Combemale P. A new treatment in scalp psoriasis: Xamiol (R) formulation and results at 8 weeks. Annales de Dermatologie et de Venereologie 2009;136(Suppl 3):S42‐5.
-
- Jemec GBE, Burden D, Poulin Y, Ortonne JP. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients and the vehicle [Abstract P2733.] American Academy of Dermatology 65th Annual Meeting 2‐6 February 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB182. - PubMed
-
- Jemec GBE, Ganslandt C, Ortonne J‐P, Poulin Y, Burden AD, Unamuno P, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. Journal of the American Academy of Dermatology 2008; Vol. 59, issue 3:455‐63. [PUBMED: 18694678] - PubMed
Jemec 2008 (P) {published and unpublished data}
-
- Combemale P. A new treatment in scalp psoriasis: Xamiol (R) formulation and results at 8 weeks. Annales de Dermatologie et de Venereologie 2009;136(Suppl 3):S42‐5.
-
- Jemec GBE, Burden D, Poulin Y, Ortonne JP. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients and the vehicle [Abstract P2733.] American Academy of Dermatology 65th Annual Meeting 2‐6 February 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB182. - PubMed
-
- Jemec GBE, Ganslandt C, Ortonne J‐P, Poulin Y, Burden AD, Unamuno P, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. Journal of the American Academy of Dermatology 2008; Vol. 59, issue 3:455‐63. [PUBMED: 18694678] - PubMed
Ji 2008 {published data only}
-
- Gottschalk RW, Johnson LA. Calcitriol 3 micrograms/g versus calcipotriol 50 micrograms/g for plaque psoriasis: treatment, maintenance and cost [Abstract P2720.] American Academy of Dermatology 65th Annual Meeting 2‐6 February 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB179.
-
- Ji S, Chen X, Wang B, Jin H, Zhao G, Wang Y, et al. Calcitriol ointment versus calcipotriol ointment in the treatment of psoriasis: a single‐blind, randomized, multicenter trial. Zhonghua Pifuke Zazhi (Chinese Journal of Dermatology) 2008; Vol. 41, issue 3:153‐6.
-
- Xuejun Z, Baoxi W, Guang Z, Jun G, Zhiqiang C, Briantais P, et al. A comparison of the efficacy and safety of twice daily applications of calcitriol 3 lg/g ointment versus calcipotriol 50 mcg/g ointment in subjects with mild to moderate chronic plaque‐type psoriasis. [Abstract P0647.] 14th Congress of the European Academy of Dermatology and Venereology, 12‐15th October 2005, London, UK. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):172. - PubMed
-
- Zhu X, Wang B, Zhao G, Gu J, Chen Z, Briantais P, et al. An investigator‐masked comparison of the efficacy and safety of twice daily applications of calcitriol 3 microg/g ointment vs. calcipotriol 50 microg/g ointment in subjects with mild to moderate chronic plaque‐type psoriasis. Journal of the European Academy of Dermatology & Venereology 2007; Vol. 21, issue 4:466‐72. [PUBMED: 17373972] - PubMed
Jin 2001 {published data only}
-
- Jin HZ, Wang JB, He ZX, Xie Y. A Randomized, Placebo Controlled, Double Blind Trial Study on IL‐8 Monoclonal Antibody Cream in the Treatment of Psoriasis Vulgaris. Chinese Journal of Clinical Pharmacology 2001;17(1):36‐40.
Jorizzo 1997 {published data only}
-
- Jorizzo Jl, Magee K, Stewart DM, Lebwohl MG, Rajagopalan R, Brown JJ. Clobetasol propionate emollient 0.05 percent: hypothalamic‐pituitary‐adrenal‐axis safety and four‐week clinical efficacy results in plaque‐type psoriasis. Cutis 1997;60(1):55‐60. [PUBMED: 9252738] - PubMed
Jorizzo 2007 {published data only (unpublished sought but not used)}
-
- Jorizzo J. Efficacy with calcipotriene and betamethasone dipropionate ointment in patients with moderate and severe psoriasis [Abstract P2780.] American Academy of Dermatology 65th Annual Meeting 2‐6 February, 2007. Journal of the American Academy of Dermatology 2007; Vol. 56, issue 2:AB194.
Kang 1998 {published and unpublished data}
-
- Kang S, Yi S, Griffiths CEM, Fancher L, Hamilton TA, Choi JH. Calcipotriene‐Induced Improvement in Psoriasis Is Associated with Reduced Interleukin‐8 and Increased Interleukin‐10 Levels within Lesions. British Journal Of Dermatology 1998;138(1):77‐83. [PUBMED: 9536226] - PubMed
Kanzler 1993 {published and unpublished data}
-
- Kanzler MH, Gorsulowsky DC. Efficacy of topical 5% liquor carbonis detergens vs. its emollient base in the treatment of psoriasis. British Journal Of Dermatology 1993;129(3):310‐4. [PUBMED: 8286230] - PubMed
Katz 1987a {published data only}
-
- Katz HI, Hien NT, Prawer S, Scot JC, Grivna EM. Betamethasone Dipropionate in Optimized Vehicle. Intermittent Pulse Dosing for Extended Maintenance Treatment of Psoriasis. Archives of Dermatology 1987;123(10):1308‐11. [PUBMED: 3662562] - PubMed
Katz 1991a {published data only}
-
- Katz HI, Prawer SE, Medansky RS, Krueger GG, Mooney JJ, Jones ML, et al. Intermittent corticosteroid maintenance treatment of psoriasis: a double‐blind multicenter trial of augmented betamethasone dipropionate ointment in a pulse dose treatment regimen. Dermatologica 1991;183(4):269‐74. [PUBMED: 1809589] - PubMed
Katz 1991b {published data only}
-
- Katz HI, Gross E, Buxman M, Prawer SE, Schwartzel EH, Gibson JR. A double‐blind, vehicle‐controlled paired comparison of halobetasol propionate cream on patients with plaque psoriasis. Journal of the American Academy of Dermatology 1991;25(6 Pt 2):1175‐8. [PUBMED: 1757613] - PubMed
Kaufmann 2002 (H) {published and unpublished data}
-
- Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A New Calcipotriol/Betamethasone Dipropionate Formulation (Daivobet(TM)) Is an Effective Once‐Daily Treatment for Psoriasis vulgaris. Dermatology 2002;205(4):389‐93. [PUBMED: 12444337] - PubMed
Kaufmann 2002 (P) {published and unpublished data}
-
- Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A New Calcipotriol/Betamethasone Dipropionate Formulation (Daivobet(TM)) Is an Effective Once‐Daily Treatment for Psoriasis vulgaris. Dermatology 2002;205(4):389‐93. [PUBMED: 12444337] - PubMed
Kim 1994 {published data only}
-
- Kim JY, You YH, Kim TY, Kim CW. Comparative Study of Calcipotriol and Desoxymethasone Ointments in the Treatment of Psoriasis Vulgaris. The Clinical Effect and Immunohistochemical Change. Korean Journal of Dermatology 1994;32(6):1054‐63. [EMBASE: 1995058111]
Kiss 1996 {published data only}
-
- Carder KR. A randomized double blind parallel group dose‐ranging comparison of the efficacy and safety of calcipotriene solution in the treatment of scalp psoriasis [Abstract 844]. Journal of Investigative Dermatology 1996;106(4):946.
-
- Kiss I, McCreary HL, Siskin SB, Epinette WW. A Randomized, Double‐ Blind, Parallel Group, Dose‐Ranging Comparison of the Efficacy and Safety of Calcipotriene Solution in the Treatment of Scalp Psoriasis. 54th Annual Meeting of the American Academy of Dermatology, 10‐15 February 1996. Journal of the American Academy of Dermatology. 1996:P301.
Klaber 1994 {published data only}
-
- Klaber MR, Hutchinson PE, Pedvis‐Leftick A, Kragballe K, Reunala TL, Kerkhof PC, et al. Comparative effects of calcipotriol solution (50 micrograms/ml) and betamethasone 17‐valerate solution (1 mg/ml) in the treatment of scalp psoriasis. British Journal Of Dermatology 1994;131(5):678‐83. [PUBMED: 7999600] - PubMed
Klaber 2000b {published and unpublished data}
-
- Klaber MR, McKinnon C. Calcipotriol (DovonexR) scalp solution in the treatment of scalp psoriasis: Comparative efficacy with 1% coal tar/1% coconut oil/0.5% salicylic acid (CapasalR) shampoo, and long‐term experience. Journal Of Dermatological Treatment 2000;11(1):21‐8. [EMBASE: 2000114933]
Koo 2006 {published data only (unpublished sought but not used)}
-
- Koo J, Blum RR, Lebwohl M. A randomized, multicenter study of calcipotriene ointment and clobetasol propionate foam in the sequential treatment of localized plaque‐type psoriasis: short‐ and long‐term outcomes. Journal of the American Academy of Dermatology 2006; Vol. 55, issue 4:637‐41. [PUBMED: 17010744] - PubMed
Köse 1997 {published data only}
-
- Köse O. Calcipotriol ointment vs clobetasol solution in scalp psoriasis. Journal Of Dermatological Treatment 1997;8:287. [EMBASE: 1998022654]
Kragballe 1988b {published and unpublished data}
-
- Kragballe K, Beck HI, Søgaard H. Improvement of Psoriasis by a Topical Vitamin D3 Analogue (Mc 903) in a Double‐Blind Study. British Journal Of Dermatology 1988;119(2):223‐30. [EMBASE: 1988203615] - PubMed
Kragballe 1991a {published and unpublished data}
-
- Kragballe K, Gjertsen BT, Hoop D, Karlsmark T, Kerkhof PC, Larko O, et al. Double‐blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet 1991;337(8735):193‐6. [PUBMED: 1670840] - PubMed
Kragballe 1998b {published and unpublished data}
-
- Glade CP, Erp PE, Kerkhof PC. Epidermal cell DNA content and intermediate filaments keratin 10 and vimentin after treatment of psoriasis with calcipotriol cream once daily, twice daily and in combination with clobetasone 17‐butyrate cream or betamethasone 17‐valerate cream: a comparative flow cytometric study. British Journal Of Dermatology 1996;135(3):379‐84. [PUBMED: 8949429] - PubMed
-
- Kragballe K, Barnes L, Hamberg KJ, Hutchinson P, Murphy F, Moller S, et al. Calcipotriol Cream with or without Concurrent Topical Corticosteroid in Psoriasis: Tolerability and Efficacy. British Journal Of Dermatology 1998;139(4):649‐54. [MEDLINE: ] - PubMed
-
- Kerkhof PC. Calcipotriol cream and concurrent corticosteroids in psoriasis. Journal of the European Academy of Dermatology & Venereology 1995; Vol. 5, issue Suppl 1:S184.
Kragballe 2004 {published and unpublished data}
-
- Kragballe K, Noerrelund KL, Lui H, Ortonne JP, Wozel G, Uurasmaa T, et al. Efficacy of once‐daily treatment regimens with calcipotriol/betamethasone dipropionate ointment and calcipotriol ointment in psoriasis vulgaris. British Journal of Dermatology 2004;150(6):1167‐73. [PUBMED: 15214905] - PubMed
Kragballe 2006 {published and unpublished data}
-
- Gold LS. Correlation between amount of drug used versus efficacy of calcipotriene/betamethasone in severe psoriasis during a 52‐week study [Abstract P2873.] American Academy of Dermatology 64th Annual Meeting 3‐7 March, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB216.
-
- Kragballe K, Austad J, Barnes L, Bibby A, Brassinne M, Cambazard F, et al. A 52‐week randomized safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Dovobet/Daivobet/Taclonex) in the treatment of psoriasis vulgaris. British Journal of Dermatology 2006; Vol. 154, issue 6:1155‐60. [PUBMED: 16704648] - PubMed
-
- Kragballe K, Austad J, Barnes L, Bibby A, Brassinne M, Cambazard F, et al. Efficacy results of a 52‐week, randomised, double‐blind, safety study of a calcipotriol/betamethasone dipropionate two‐compound product (Daivobet/Dovobet/Taclonex) in the treatment of psoriasis vulgaris. Dermatology 2006; Vol. 213, issue 4:319‐26. [PUBMED: 17135738] - PubMed
-
- Kragballe K, Bibby A. Long‐term efficacy of a calcipotriene/betamethasone dipropionate two compound product in Psoriasis vulgaris [Abstract P2891] American Academy of Dermatology 64th Annual Meeting 3‐7 March, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB220.
Kragballe 2009 {published and unpublished data}
-
- Kragballe K, Ganslandt C, Ortonne J P. A calcipotriol plus betamethasone dipropionate scalp formulation significantly reduces itching in scalp psoriasis. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB172.
-
- Kragballe K, Hoffmann V, Ortonne J P, Tan J, Nordin P, Segaert S. Efficacy and safety of calcipotriol plus betamethasone dipropionate scalp formulation compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized controlled trial. British Journal of Dermatology 2009;161(1):159‐66. [PUBMED: 19416259] - PubMed
-
- Ortonne JP, Ganslandt C, Tan J, Nordin P, Kragballe K, Segaert S. Quality of life in patients with scalp psoriasis treated with calcipotriol/betamethasone dipropionate scalp formulation: a randomized controlled trial. Journal of the European Academy of Dermatology & Venereology 2009;23(8):919‐26. - PubMed
Kreuter 2006 (H) {published data only (unpublished sought but not used)}
-
- Kreuter A, Sommer A, Hyun J, Brautigam M, Brockmeyer NH, Altmeyer P, et al. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: a double‐blind, randomized controlled study. Archives of Dermatology 2006; Vol. 142, issue 9:1138‐43. [PUBMED: 16983001] - PubMed
Kreuter 2006 (P) {published data only (unpublished sought but not used)}
-
- Kreuter A, Sommer A, Hyun J, Brautigam M, Brockmeyer NH, Altmeyer P, et al. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: a double‐blind, randomized controlled study. Archives of Dermatology 2006; Vol. 142, issue 9:1138‐43. [PUBMED: 16983001] - PubMed
Krueger 1998 {published data only}
-
- Krueger GG, Drake LA, Elias PM, Lowe NJ, Guzzo C, Weinstein GD, et al. The safety and efficacy of tazarotene gel, a topical acetylenic retinoid, in the treatment of psoriasis. Archives of Dermatology 1998;134(1):57‐60. [PUBMED: 9449910] - PubMed
Lahfa 2003 {published data only (unpublished sought but not used)}
-
- Lahfa M, Mrowietz U, Koenig M, Simon JC. Calcitriol ointment and clobetasol propionate cream: a new regimen for the treatment of plaque psoriasis. European Journal of Dermatology 2003; Vol. 13, issue 3:261‐5. - PubMed
Landi 1993 {published data only}
-
- Landi G. Efficacy and safety of calcipotriol ointment compared to clobetasol ointment in psoriasis vulgaris. 3rd Congress of the European Academy of Dermatology & Venereology. Copenhagen, Denmark, 1993:199.
-
- Landi G, Pierleoni M, Polverelli M, Fioravanti F. Calcipotriol, a New Topical Product in the Therapy of Psoriasis: Controlled Study Versus Clobetasol [Il Calcipotriol, Nuovo Topico Nella Terapia Della Psoriasi: Studio Controllato Versus Clobetasol]. Giornale Italiano di Dermatologia e Venereologia 1993;128(9):89‐93. [EMBASE: 1993360641]
Lane 1983 {published data only}
-
- Lane AT, Wachs GN, Weston WL. Once‐Daily Treatment of Psoriasis with Topical Glucocorticosteroid Ointments. Journal of the American Academy of Dermatology 1983;8(4):523‐5. [PUBMED: 6853785] - PubMed
Langley 2011 (H) {published and unpublished data}
-
- Langley R, Gupta A, Papp K, Wexler D, Østerdal M, Ćurčić D. Calcipotriol plus Betamethasone Dipropionate Gel Compared with Tacalcitol Ointment and the Gel Vehicle Alone in Patients with Psoriasis Vulgaris: A Randomized, Controlled Clinical Trial. Dermatology 2011; Vol. 222, issue 2:148‐56. [PUBMED: 21293107] - PubMed
-
- Langley R, Gupta A, Wexler D, Curcic D, Papp K. Efficacy and safety of calcipotriene plus betamethasone dipropionate gel compared to tacalcitol ointment and gel vehicle alone in patients with psoriasis vulgaris: A randomized controlled trial. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB134. - PubMed
-
- NCT00670241. Efficacy and Safety of Calcipotriol Plus Betamethasone Dipropionate Gel Compared With Tacalcitol Ointment and the Gel Vehicle Alone in Patients With Psoriasis Vulgaris. clinicaltrials.gov/ct2/results?term=NCT00670241 (accessed 2010).
Langley 2011 (P) {published and unpublished data}
-
- Langley R, Gupta A, Papp K, Wexler D, Østerdal M, Ćurčić D. Calcipotriol plus Betamethasone Dipropionate Gel Compared with Tacalcitol Ointment and the Gel Vehicle Alone in Patients with Psoriasis Vulgaris: A Randomized, Controlled Clinical Trial. Dermatology 2011; Vol. 222, issue 2:148‐56. [PUBMED: 21293107] - PubMed
-
- Langley R, Gupta A, Wexler D, Curcic D, Papp K. Efficacy and safety of calcipotriene plus betamethasone dipropionate gel compared to tacalcitol ointment and gel vehicle alone in patients with psoriasis vulgaris: A randomized controlled trial. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB134. - PubMed
-
- NCT00670241. Efficacy and Safety of Calcipotriol Plus Betamethasone Dipropionate Gel Compared With Tacalcitol Ointment and the Gel Vehicle Alone in Patients With Psoriasis Vulgaris. clinicaltrials.gov/ct2/results?term=NCT00670241 (accessed 2010).
Langner 1992 {published data only}
-
- Langner A, Verjans H, Stapor V, Mol M, Fraczykowska M. 1 Alpha 25‐Dihydroxyvitamin D‐3 (Calcitriol) Ointment in Psoriasis. Journal Of Dermatological Treatment 1992;3(4):177‐80. [EMBASE: 1993020253]
Langner 1993 {published data only}
-
- Langner A, Verjans H, Stapor V, Mol M, Fraczykowska M. Topical Calcitriol in the Treatment of Chronic Plaque Psoriasis: A Double‐Blind Study. British Journal Of Dermatology 1993;128(5):566‐71. [PUBMED: 8504051] - PubMed
Langner 2001 (H) {published data only}
-
- Langner A, Stapor W, Ambroziak M. Efficacy and tolerance of topical calcitriol 3 microg g(‐1) in psoriasis treatment: a review of our experience in Poland. British Journal Of Dermatology 2001;58:11‐16. [PUBMED: 11501507] - PubMed
Langner 2001 (P) {published data only}
-
- Langner A, Stapor W, Ambroziak M. Efficacy and tolerance of topical calcitriol 3 microg g(‐1) in psoriasis treatment: a review of our experience in Poland. British Journal Of Dermatology 2001;58:11‐16. [PUBMED: 11501507] - PubMed
Lassus 1991 {published data only}
-
- Lassus A, Forsstrom S. A double‐blind study comparing oleum horwathiensis with placebo in the treatment of psoriasis. Journal of International Medical Research 1991;19(2):137‐46. [PUBMED: 1864450] - PubMed
Lebwohl 2002 {published data only}
-
- Lebwohl M, Scher RK, Washenik K, Krueger G, Menter A, Koo J, et al. A randomized, double‐blind placebo‐controlled study of the safety and efficacy of clobetasol propionate 0.05% foam in the treatment of non‐scalp psoriasis. Skin Pharmacology & Applied Skin Physiology 2001;14:168.
-
- Lebwohl M, Sherer D, Washenik K, Krueger GG, Menter A, Koo J, Feldman SR. A randomized, double‐blind, placebo‐controlled study of clobetasol propionate 0.05% foam in the treatment of nonscalp psoriasis. International Journal Of Dermatology 2002;41(5):269‐74. [PUBMED: 12100701] - PubMed
Lebwohl 2004 {published data only (unpublished sought but not used)}
-
- Lebwohl M, Freeman AK, Chapman MS, Feldman SR, Hartle JE, Henning A. Tacrolimus ointment is effective for facial and intertriginous psoriasis. Journal of the American Academy of Dermatology 2004;51(5):723‐30. [PUBMED: 15523350] - PubMed
Lebwohl 2007 {published and unpublished data}
-
- Lebwohl M, Menter A, Weiss J, Clark SD, Flores J, Powers J, et al. Calcitriol 3 microg/g ointment in the management of mild to moderate plaque type psoriasis: results from 2 placebo‐controlled, multicenter, randomized double‐blind, clinical studies. Journal of Drugs in Dermatology 2007; Vol. 6, issue 4:428‐35. [PUBMED: 17668541] - PubMed
Lee 2007 {published data only}
-
- Lee J, Youn J, Kim N, Kim K, Kim T, Choi J, et al. A randomized investigator‐blinded comparative study of calcitriol twice a day vs. diflucortolone valerate morning plus calcitriol evening application in the treatment of mild to moderate psoriasis. Journal of the European Academy of Dermatology & Venereology 2007; Vol. 21, issue Suppl 1:19.
Lepaw 1978 {published data only}
-
- Lepaw MI. Double‐Blind Comparison of Halcinonide Solution and Placebo Control in Treatment of Psoriasis of the Scalp. Cutis 1978;21(4):571‐3. [PUBMED: 346315] - PubMed
Levine 2010 (H) {published and unpublished data}
-
- Levine D, Even‐Chen Z, Lipets I, Pritulo OA, Svyatenko TV, Andrashko Y, et al. Pilot, multicenter, double‐blind, randomized placebo‐controlled bilateral comparative study of a combination of calcipotriene and nicotinamide for the treatment of psoriasis. Journal of the American Academy of Dermatology 2010;63(5):775‐81. [PUBMED: 20599292] - PubMed
Levine 2010 (P) {published and unpublished data}
-
- Levine D, Even‐Chen Z, Lipets I, Pritulo OA, Svyatenko TV, Andrashko Y, et al. Pilot, multicenter, double‐blind, randomized placebo‐controlled bilateral comparative study of a combination of calcipotriene and nicotinamide for the treatment of psoriasis. Journal of the American Academy of Dermatology 2010;63(5):775‐81. [PUBMED: 20599292] - PubMed
Liao 2007 {published and unpublished data}
-
- Liao YH, Chiu HC, Tseng YS, Tsai TF. Comparison of cutaneous tolerance and efficacy of calcitriol 3 microg g(‐1) ointment and tacrolimus 0.3 mg g(‐1) ointment in chronic plaque psoriasis involving facial or genitofemoral areas: a double‐blind, randomized controlled trial. British Journal of Dermatology 2007; Vol. 157, issue 5:1005‐12. [PUBMED: 17935517] - PubMed
Lin 2007 {published and unpublished data}
-
- Lin YK, Wong WR, Chang YC, Chang CJ, Tsay PK, Chang SC, et al. The efficacy and safety of topically applied indigo naturalis ointment in patients with plaque‐type psoriasis. Dermatology 2007;214(2):155‐61. [PUBMED: 17341866] - PubMed
Lin 2008 {published data only}
-
- Lin YK, Chang CJ, Chang YC, Wong WR, Chang SC, Pang JHS. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer‐blind, vehicle‐controlled trial using indigo naturalis. Archives of Dermatology 2008;144(11):1457‐64. [PUBMED: 19015420] - PubMed
Lister 1997 {published data only}
-
- Lister RK, Woodrow Sl, Hughes JH, Cerio R, Norris PG, Griffiths CEM, et al. Can dithranol have a lasting effect and be more acceptable to patients? Micanol Cream ‐ a trial of 171 patients with psoriasis. British Journal of Dermatology 1997;137(Suppl 50):17.
Lowe 2005 {published data only}
-
- Lowe N, Feldman SR, Sherer D, Weiss J, Shavin JS, Lin YL, et al. Clobetasol propionate lotion, an efficient and safe alternative to clobetasol propionate emollient cream in subjects with moderate to severe plaque‐type psoriasis. Journal of Dermatological Treatment 2005; Vol. 16, issue 3:158‐64. [PUBMED: 16096182] - PubMed
Luger 2008 {published and unpublished data}
-
- Cambazard F. Xamiol, a new treatment in scalp psoriasis: short‐ and long‐term results [Un nouveau traitement dans le psoriasis du cuir chevelu : Xamiol : Resultats court terme et a long terme]. Annales de Dermatologie et de Venereologie 2009;136(Suppl 3):546‐50.
-
- Luger T, Kidson P, Cambazard F, Larsen FG. A 1‐year, randomized, double‐blind safety study of long‐term treatment of a new gel formulation containing calcipotriene plus betamethasone dipropionate in scalp psoriasis. Journal of the American Academy of Dermatology 2008; Vol. 58, issue 2:AB134.
Maier 2004 {published data only}
-
- Maier H. Prospective, randomized controlled double‐blind study on the efficacy and safety of a series of herbal skin‐care products for stable chronic plaque psoriasis [Abstract P061] European Congress on Psoriasis 2004. Journal of the European Academy of Dermatology & Venereology 2004;18(6):794.
Medansky 1987 {published data only}
-
- Medansky RS, Brody NI, Kanof NB, Russo GJ, Peets EA. Clinical Investigations of Mometasone Furoate ‐ a Novel Nonfluorinated, Topical Corticosteroid. Seminars In Dermatology 1987;6(2):94‐100. [EMBASE: 1988051740]
Medansky 1996 {published data only}
-
- Medansky RS, Greenspan A, Kraus SJ, Todd Plott R. The Comparative Efficacy of Diflorasone Diacetate Ointment 0.05% (Psorcon®) vs Calcipotriene Ointment (Dovonex®) in the Treatment of Psoriasis. Journal of Geriatric Dermatology 1996;4(1):20‐24.
Menter 2009 {published and unpublished data}
-
- Menter A, Abramovits W, Colon LE, Johnson LA, Gottschalk RW. Comparing clobetasol propionate 0.05% spray to calcipotriene 0.005% betamethasone dipropionate 0.064% ointment for the treatment of moderate to severe plaque psoriasis. Journal of Drugs in Dermatology 2009;8(1):52‐7. [PUBMED: 19180896] - PubMed
-
- Menter A, Colon L, Johnson L, Gottschalk R. Results from a randomized study comparing clobetasol propionate 0.05% spray to calcipotriene 0.005%, betamethasone dipropionate 0.064% ointment for the treatment of plaque psoriasis. Journal of the American Academy of Dermatology 2008; Vol. 58, issue 2:AB124.
Molin 1997 {published and unpublished data}
-
- Molin L, Cutler TP, Helander I, Nyfors B. Calcipotriol cream and betamethasone valerate cream of equal efficacy in psoriasis. Journal of the European Academy of Dermatology & Venereology 1995;5(Suppl 1):S92.
-
- Molin L, Cutler TP, Helander I, Nyfors B. Comparative Efficacy of Calcipotriol Cream and Betamethasone Valerate Cream in the Treatment of Psoriasis. Journal of Investigative Dermatology. Symposium Proceedings 1996;1(1):110. - PubMed
-
- Molin L, Cutler TP, Helander I, Nyfors B, Downes N. Comparative Efficacy of Calcipotriol (Mc903) Cream and Betamethasone 17‐Valerate Cream in the Treatment of Chronic Plaque Psoriasis. A Randomized, Double‐Blind, Parallel Group Multicentre Study. Calcipotriol Study Group. British Journal Of Dermatology 1997;136(1):89‐93. [PUBMED: 9039301] - PubMed
Monastirli 2000 {published data only}
-
- Monastirli A, Zografakis CH, Braun H, Pasmatzi E, Georgiou S, Sakkis TH, et al. Calcipotriol vs. Anthralin in the treatment of chronic plaque psoriasis. H+G Zeitschrift Fur Hautkrankheiten. 2000;75(11):626‐9. [EMBASE: 2000428558]
Mortensen 1993b {published and unpublished data}
-
- Mortensen L, Kragballe K, Wegmann E, Schifter S, Risteli J, Charles P. Treatment of Psoriasis Vulgaris with Topical Calcipotriol Has No Short‐Term Effect on Calcium or Bone Metabolism. A Randomized, Double‐Blind, Placebo‐Controlled Study. Acta Dermato Venereologica 1993;73(4):300‐4. [PUBMED: 7904106] - PubMed
Olsen 1991 {published data only}
-
- Olsen EA, Cram DL, Ellis CN, Hickman JG, Jacobson C, Jenkins EE, et al. A double‐blind, vehicle‐controlled study of clobetasol propionate 0.05% (Temovate) scalp application in the treatment of moderate to severe scalp psoriasis. Journal of the American Academy of Dermatology 1991;24(3):443‐7. [PUBMED: 2061442] - PubMed
Olsen 1996 (1) {published data only}
-
- Olsen EA. Efficacy and Safety of Fluticasone Propionate 0.005% Ointment in the Treatment of Psoriasis. Cutis 1996;57(2 Suppl):57‐61. [PUBMED: 8646872] - PubMed
Olsen 1996 (2) {published data only}
-
- Olsen EA. Efficacy and Safety of Fluticasone Propionate 0.005% Ointment in the Treatment of Psoriasis. Cutis 1996;2 Suppl:57‐61. [PUBMED: 8646872] - PubMed
Oranje 1997 {published data only}
-
- Oranje AP, Marcoux DSA, Prendiville J, Krafchik B, Toole J, Rosenthal D, et al. Topical calcipotriol in childhood psoriasis. Journal of the American Academy of Dermatology 1997;36(2 Pt 1):203‐8. [PUBMED: 9039169] - PubMed
Ormerod 1997 {published and unpublished data}
-
- Ormerod AD, Dwyer CM, Weller R, Cox DH, Price R. A comparison of subjective and objective measures of reduction of psoriasis with the use of ultrasound, reflectance colorimetry, computerized video image analysis, and nitric oxide production. Journal of the American Academy of Dermatology 1997;37(1):51‐7. [PUBMED: 9216523 ] - PubMed
Ormerod 2000 {published data only}
-
- Ormerod AD, Copeland P, Shah SA. Treatment of psoriasis with topical NG‐monomethyl‐L‐arginine, an inhibitor of nitric oxide synthesis. British Journal Of Dermatology 2000;142(5):985‐90. [PUBMED: 10809860] - PubMed
Ormerod 2005 {published data only}
-
- Ormerod AD, Shah SA, Copeland P, Omar G, Winfield A. Treatment of psoriasis with topical sirolimus: preclinical development and a randomized, double‐blind trial. British Journal of Dermatology 2005;152(4):758‐64. [PUBMED: 15840110 ] - PubMed
Ortonne 1994 {published data only}
-
- Ortonne JP, Bazex J, Binet O, Bombart M, Brun P, Carreau O, et al. Psoriasis: new Therapeutic Modality by Calcipotriol and Betamethasone Dipropionate. Nouvelles Dermatologiques 1994;13(10):746‐51. [EMBASE: 1995010294]
Ortonne 2003 {published data only}
-
- Nicolas JF. Calcitriol vs calcipotriol in psoriasis of sensitive areas [Abstract]. 20th World Congress of Dermatology 1‐5 July 2002, Paris. Paris, 2002:SA1048.
-
- Ortonne JP, Humbert P, Nicolas JF, Tsankov N, Tonev SD, Janin A, et al. Intra‐individual comparison of the cutaneous safety and efficacy of calcitriol 3 microg g(‐1) ointment and calcipotriol 50 microg g(‐1) ointment on chronic plaque psoriasis localized in facial, hairline, retroauricular or flexural areas. British Journal of Dermatology 2003;148(2):326‐33. [PUBMED: 12588387] - PubMed
Ortonne 2004 {published and unpublished data}
-
- Ortonne JP, Kaufmann R, Lecha M, Goodfield M. Efficacy of treatment with calcipotriol/betamethasone dipropionate followed by calcipotriol alone compared with tacalcitol for the treatment of psoriasis vulgaris: A randomised, double‐blind trial. Dermatology 2004;209(4):308‐13. [PUBMED: 15539894] - PubMed
-
- Ortonne JP, Traulsen J, Kaufmann R, Lecha M, Goodfield M. Calcipotriol/betamethasone dipropionate ointment once daily versus tacalcitol once daily in psoriasis vulgaris [Abstract P27‐49.] 12th Congress of the European Academy of Dermatology and Venereology. 15‐18 October 2003, Barcelona, Spain. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 3):374.
Ortonne 2006 {published data only}
-
- Ortonne JP, Kerkhof PCM, Prinz JC, Bieber T, Lahfa M, Rubins A, et al. 0.3% Tacrolimus gel and 0.5% Tacrolimus cream show efficacy in mild to moderate plaque psoriasis: Results of a randomized, open‐label, observer‐blinded study. Acta Dermato‐Venereologica 2006; Vol. 86, issue 1:29‐33. [PUBMED: 16585986] - PubMed
-
- Vissers W H, Vlijmen I, Erp P E, Jong E M, Kerkhof P C. Topical treatment of mild to moderate plaque psoriasis with 0.3% tacrolimus gel and 0.5% tacrolimus cream: the effect on SUM score, epidermal proliferation, keratinization, T‐cell subsets and HLA‐DR expression. British Journal of Dermatology 2008;158(4):705‐12. - PubMed
Ortonne 2010 {published and unpublished data}
-
- Ortonne JP, Noerrelund KL, Papp K, Herpe L, Sebastian M, Herrera E, et al. Comparison of two different dose combinations of calcipotriol/hydrocortisone ointment used once daily for the treatment of psoriasis vulgaris on the face and body. European Journal of Dermatology 2010;20(5):585‐9. [PUBMED: 20732849] - PubMed
Papakostantinou 2005 {published data only}
-
- Papakostantinou E, Xenos K, Markantonis S L, Druska S, Stratigos A, Katsambas A. Efficacy of 2 weeks' application of theophylline ointment in psoriasis vulgaris. Journal of Dermatological Treatment 2005;16(3):169‐70. [PUBMED: 16096184] - PubMed
Papp 2003 (H) {published and unpublished data}
-
- Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. Journal of the American Academy of Dermatology 2003;48(1):48‐54. [PUBMED: 12522370] - PubMed
Papp 2003 (P) {published and unpublished data}
-
- Papp KA, Guenther L, Boyden B, Larsen FG, Harvima RJ, Guilhou JJ, et al. Early onset of action and efficacy of a combination of calcipotriene and betamethasone dipropionate in the treatment of psoriasis. Journal of the American Academy of Dermatology 2003;48(1):48‐54. [PUBMED: 12522370] - PubMed
Pariser 1996 {published data only}
-
- Pariser DM, Pariser RJ, Breneman D, Lebwohl M, Kalb R, Moore J, et al. Calcipotriene ointment applied once a day for psoriasis: A double‐ blind, multicenter, placebo‐controlled study. Archives of Dermatology 1996;132(12):1527. [PUBMED: 8961897] - PubMed
Pauporte 2004 {published and unpublished data}
-
- Pauporte M, Maibach H, Lowe N, Pugliese M, Friedman DJ, Mendelsohn H, et al. Fluocinolone acetonide topical oil for scalp psoriasis. Journal of Dermatological Treatment 2004;15(6):360‐4. [PUBMED: 15764047] - PubMed
Perez 1996 {published and unpublished data}
-
- Perez A, Chen TC, Turner A, Raab R, Bhawan J, Poche P, et al. Efficacy and Safety of Topical Calcitriol (1,25‐Dihydroxyvitamin D3) for the Treatment of Psoriasis. British Journal Of Dermatology 1996;134(2):238‐46. [PUBMED: 8746336] - PubMed
Pinheiro 1997 {published and unpublished data}
-
- Pinheiro N. Comparative Effects of Calcipotriol Ointment (50 Micrograms/G) and 5% Coal Tar/2% Allantoin/0.5% Hydrocortisone Cream in Treating Plaque Psoriasis. British Journal of Clinical Practice 1997;51(1):16‐9. [PUBMED: 9158266] - PubMed
Poulin 2010 {published and unpublished data}
-
- Poulin Y, Papp K, Bissonnette R, Barber K, Kerrouche N, Villemagne H, et al. Clobetasol propionate shampoo 0.05% is efficacious and safe for long‐term control of moderate scalp psoriasis. Journal of Dermatological Treatment 2010;21(3):185‐92. [PUBMED: 20131980] - PubMed
-
- Poulin Y, Papp K, Bissonnette R, Guenther L, Tan J, Lynde C, et al. Clobetasol propionate shampoo 0.05% is efficacious and safe for long‐term control of scalp psoriasis. Cutis 2010;85(1):43‐50. [PUBMED: 20184211] - PubMed
-
- Poulin Y, Papp K, Guenther L, Bissonnette R. Twice‐weekly use of clobetasol propionate 0.05% shampoo is effective and safe for the long‐term control of scalp psoriasis. Journal of the American Academy of Dermatology 2010; Vol. 62, issue 3 Suppl 1:AB132. [0190‐9622]
Powers 2005 {published and unpublished data}
-
- Powers J. Assessment of the Efficacy and Safety of Calcitriol 3mcg/g Ointment in the Treatment of Chronic Plaque Psoriasis. 7th Asian Congress of Dermatology incorporating the 5th Regional Conference of Paediatric Dermatology Kuala Lumpur, Malaysia 28 September ‐1 October, 2005 2005:367.
-
- Powers J, Balin AK, Kempers S, Glinert RJ, Fleming T, Graeber M. Assessment of the efficacy and safety of calcitriol 3ugg‐1 ointment in the treatment of chronic plaque psoriasis [Abstract P‐40.] 85th BAD Annual Meeting 5‐8 July 2005, Glasgow, UK. British Journal of Dermatology 2005; Vol. 153, issue Suppl 1:32.
Reygagne 2005 {published and unpublished data}
-
- Reygagne P, Mrowietz U, Decroix J, Waard‐Van Der Spek FB, Acebes LO, Figueiredo A, et al. Clobetasol propionate shampoo 0.05% and calcipotriol solution 0.005%: A randomized comparison of efficacy and safety in subjects with scalp psoriasis. Journal of Dermatological Treatment 2005; Vol. 16, issue 1:31‐6. [PUBMED: 15897165] - PubMed
-
- Reygagne P, Mrowietz U, Decroix J, Spek W, Olmos Acebes L, Figueiredo A, et al. Four‐week efficacy and safety comparison of a new clobetasol shampoo and calcipotriol solution 0.005% in subjects with scalp psoriasis. 11th Congress of the European Academy of Dermatology & Venereology, 2‐6 October, 2002, Prague. 2002:27‐36.
Ruzicka 1998 {published data only}
-
- Ruzicka T, Kallinschnigg G, Lorenz B, Schroder G. Clinical Efficacy of a Monotherapy with Calcipotriol‐Ointment Compared to Combination Therapy with Calcipotriol Ointment and Betamethasone Valerate Ointment in Psoriasis Vulgaris. Journal of Investigative Dermatology 1996;1(1):107.
-
- Ruzicka T, Lorenz B. Comparison of Calcipotriol Monotherapy and a Combination of Calcipotriol and Betamethasone Valerate after 2 Weeks' Treatment with Calcipotriol in the Topical Therapy of Psoriasis Vulgaris: A Multicentre, Double‐Blind, Randomized Study. British Journal Of Dermatology 1998;138(2):254‐8. [PUBMED: 9602870] - PubMed
Salmhofer 2000 {published data only}
-
- Salmhofer W, Maier H, Soyer HP, Honigsmann H, Hodl S. Double‐blind, placebo‐controlled, randomized, right‐left study comparing calcipotriol monotherapy with a combined treatment of calcipotriol and diflucortolone valerate in chronic plaque psoriasis. Acta Dermato Venereologica 2000;Suppl 211:5‐8. [PUBMED: 11234559] - PubMed
Sanchez 2001 {published and unpublished data}
-
- Sanchez RM, Iglesias SM, Umbert MP. Treatment of plague‐type psoriasis with topical propylthiouracil. Actas Dermo Sifiliograficas 2001;92(4):174‐6. [EMBASE: 2001206374]
Santoianni 2001 {published data only}
-
- Santoianni P, Iorio S, Giannetti A, Manfredi G, Rebora A, Landi G, et al. Short‐term clinical efficacy of the association betamethasone 17‐valerate 21‐acetate + tretinoine + salicylic acid in outpatients affected with disseminated keratotic plaque psoriasis: A double blinded placebo‐controlled multicentric randomized clinical trial. Giornale Italiano di Dermatologia e Venereologia 2001;136(1):77‐83. [EMBASE: 2001160666]
Saraceno 2007 {published data only (unpublished sought but not used)}
-
- Saraceno R, Andreassi L, Ayala F, Bongiorno MR, Giannetti A, Lisi P, et al. Efficacy, safety and quality of life of calcipotriol/betamethasone dipropionate (Dovobet) versus calcipotriol (Daivonex) in the treatment of psoriasis vulgaris: a randomized, multicentre, clinical trial. Journal of Dermatological Treatment 2007; Vol. 18, issue 6:361‐5. [PUBMED: 17934937] - PubMed
Scarpa 1994 {published data only}
-
- Scarpa C. Calcipotriol: Clinical Trial Versus Betamethasone Dipropionate + Salicylic Acid. Acta Dermato Venereologica 1994;186(Suppl):47. [PUBMED: 8073835] - PubMed
Scarpa 1996 {published data only}
-
- Scarpa C. Tacalcitol Ointment Is an Efficacious and Well Tolerated Treatment for Psoriasis. Journal of the European Academy of Dermatology & Venereology 1996;6(2):142‐6. [EMBASE: 1996096733]
-
- Scarpa C, Kokelj F, Plozzer C, Lavaroni G. Efficacy and Tolerability of Tacalcitol Ointment on Psoriatic Skin: Study in 63 Patients. Journal of Investigative Dermatology. Symposium Proceedings 1996;1(1):110.
Scarpa 1997 {published data only}
-
- Scarpa C, Kokelj F, Plozzer C, Lavaroni G, Torsello P. Efficacy and Tolerability of Tacalcitol Administered Once Daily in the Treatment of Psoriasis Vulgaris (Double‐Blind, Randomized, Placebo Controlled Italian Multicenter Study). Giornale Italiano di Dermatologia e Venereologia 1997;132(5):335‐8. [EMBASE: 1997363332]
Sears 1997 {published data only}
-
- Sears HW, Bailer JW, Yeadon A. A Double‐Blind, Randomized, Placebo‐Controlled Evaluation of the Efficacy and Safety of Hydrocortisone Buteprate 0.1% Cream in the Treatment of Psoriasis. Advances In Therapy 1997;14(3):140‐9. [EMBASE: 1997223749]
Seidenari 1997 (H) {published data only}
-
- Seidenari S, Magni R, Giannetti A. Assessment of the Activity of Tacalcitol on Psoriatic Plaques by Means of Colorimetry and High‐Frequency Ultrasound: A Double‐Blind Intrasubject Half‐Side Right‐Left Comparison with Betamethasone Valerate and Placebo. Skin Pharmacology 1997;10(1):40‐7. [EMBASE: 1997139265]
Seidenari 1997 (P) {published data only}
-
- Seidenari S, Magni R, Giannetti A. Assessment of the Activity of Tacalcitol on Psoriatic Plaques by Means of Colorimetry and High‐Frequency Ultrasound: A Double‐Blind Intrasubject Half‐Side Right‐Left Comparison with Betamethasone Valerate and Placebo. Skin Pharmacology 1997;10(1):40‐7. [EMBASE: 1997139265]
Shuttleworth 1998 {published data only}
-
- Shuttleworth D, Galloway DB, Boorman GC, Donald AE. A double‐blind, placebo‐controlled study of the clinical efficacy of ciclopirox olamine (1.5%) shampoo for the control of scalp psoriasis. Journal Of Dermatological Treatment 1998;9(3):163‐7. [EMBASE: 1998346163]
Staberg 1989 {published data only}
-
- Staberg B, Roed‐Petersen J, Menne T. Efficacy of Topical Treatment in Psoriasis with Mc903, a New Vitamin D Analogue. Acta Dermato Venereologica 1989;69(2):147‐50. [PUBMED: 2564233] - PubMed
Stein 2001 {published data only}
-
- Stein LF, Sherr A, Solodkina G, Gottlieb AB, Chaudhari U. Betamethasone valerate foam for treatment of nonscalp psoriasis. Journal of Cutaneous Medicine & Surgery 2001;5(4):303‐7. [PUBMED: 11907840] - PubMed
Stuecker 2001 {published and unpublished data}
-
- Hoffmann M, Memmel U, Altmeyer P, Stuecker M. Topical treatment of chronic‐stationary plaque psoriasis with vitamin B12. Zeitschrift Fur Hautkrankheiten 2001;Suppl 1(76):S12.
-
- Stuecker M, Memmel U, Hoffmann M, Hartung J, Altmeyer P. Vitamin B(12) cream containing avocado oil in the therapy of plaque psoriasis. Dermatology 2001;203(2):141‐7. [PUBMED: 11586013] - PubMed
Stutz 1996 {published data only}
-
- Stutz JA, Ellis CN, Kang S. Failure of topical polymyxin B to improve mild plaque psoriasis [letter]. Archives of Dermatology 1996;132(2):231. [PUBMED: 8629837] - PubMed
Sudilovsky 1981 {published data only}
-
- Sudilovsky A, Muir JG, Bocobo FC. A Comparison of Single and Multiple Applications of Halcinonide Cream. International Journal Of Dermatology 1981;20(9):609‐13. [PUBMED: 7030988] - PubMed
Sutton 2001 {published data only}
-
- Sutton L, Swinehart JM, Cato A, Kaplan AS. A clinical study to determine the efficacy and safety of 1% methotrexate/Azone (MAZ) gel applied topically once daily in patients with psoriasis vulgaris. International Journal Of Dermatology 2001;40(7):464‐7. [MEDLINE: ] - PubMed
Syed 1996 {published data only}
-
- Syed TA, Ahmad SA, Holt AH, Ahmad SA, Ahmad SH, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: a placebo‐controlled, double‐blind study. Tropical Medicine & International Health 1996;1(4):505‐9. [PUBMED: 8765459] - PubMed
Syed 2001b {published data only}
-
- Syed TA, Hadi SM, Qureshi ZA, Nordstrom CG, Ali SM. Management of psoriasis vulgaris with methotrexate 0.25% in a hydrophilic gel: a placebo‐controlled, double‐blind study. Journal of Cutaneous Medicine & Surgery 2001;5(4):299‐302. [PUBMED: 11907839] - PubMed
Tham 1994 {published data only}
-
- Tham SN, Lun KC, Cheong WK. A comparative study of calcipotriol ointment and tar in chronic plaque psoriasis. British Journal Of Dermatology 1994;131(5):673‐7. [PUBMED: 7999599] - PubMed
Tyring 2010 {published and unpublished data}
-
- Tyring S, Mendoza N, Appell M, Bibby A, Foster R, Hamilton T, et al. A calcipotriene/betamethasone dipropionate two‐compound scalp formulation in the treatment of scalp psoriasis in Hispanic/Latino and Black/African American patients: Results of the randomized, 8‐week, double‐blind phase of a clinical trial. International Journal of Dermatology 2010;49(11):1328‐33. [PUBMED: 20964660] - PubMed
Tzung 2005 {published data only}
-
- Tzung TY, Wu JC, Hsu NJ, Chen YH, Ger LP. Comparison of tazarotene 0.1% gel plus petrolatum once daily versus calcipotriol 0.005% ointment twice daily in the treatment of plaque psoriasis. Acta Dermato Venereologica 2005; Vol. 85, issue 3:236‐9. [PUBMED: 16040409] - PubMed
Vali 2005 {published data only}
-
- Vali A, Asilian A, Khalesi E, Khoddami L, Shahtalebi M, Mohammady M. Evaluation of the efficacy of topical caffeine in the treatment of psoriasis vulgaris. Journal of Dermatological Treatment 2005; Vol. 16, issue 4:234‐7. [PUBMED: 216249145] - PubMed
-
- Vali A, Asilian A, Khalesi E, Khoddami L, Shahtalebi M, Mohammady M. Evaluation of the efficacy of topical caffeine in the treatment of psoriasis vulgaris: A randomized, double‐blind clinical trial [Abstract]. Iranian Journal of Dermatology 2006; Vol. 8, issue 6:87. - PubMed
Van de Kerkhof 1989 {published data only}
-
- Kerkhof PCM, Bokhoven M, Zultak M, Czarnetzki BM. A Double‐Blind Study of Topical 1 Alpha,25‐Dihydroxyvitamin D3 in Psoriasis. British Journal Of Dermatology 1989;120(5):661‐4. [PUBMED: 2667612] - PubMed
Van de Kerkhof 1996a {published data only}
-
- Kerkhof PCM, Werfel T, Haustein UF, Luger T, Czarnetzki BM, Niemann R, et al. Tacalcitol Ointment in the Treatment of Psoriasis Vulgaris: A Multicentre, Placebo‐ Controlled, Double‐ Blind Study on Efficacy and Safety. British Journal Of Dermatology 1996;135(5):758‐65. [PUBMED: 8977677] - PubMed
Van de Kerkhof 2002a {published data only}
-
- Kerkhof PCM, Green C, Hamberg KJ, Hutchinson PE, Jensen JK, Kidson P, et al. Safety and efficacy of combined high‐dose treatment with calcipotriol ointment and solution in patients with psoriasis. Dermatology 2002;204(3):214‐21. [PUBMED: 12037450] - PubMed
Van de Kerkhof 2006 {published and unpublished data}
-
- Korte J, Valk PG, Sprangers MA, Damstra RJ, Kunkeler AC, Lijnen RL, et al. A comparison of twice‐daily calcipotriol ointment with once‐daily short‐contact dithranol cream therapy: quality‐of‐life outcomes of a randomized controlled trial of supervised treatment of psoriasis in a day‐care setting. British Journal of Dermatology 2008; Vol. 158, issue 2:375‐81. - PubMed
-
- Kerkhof PC, Valk PG, Swinkels OQ, Kucharekova M, Rie MA, Vries HJ, et al. A comparison of twice‐daily calcipotriol ointment with once‐daily short‐contact dithranol cream therapy: a randomized controlled trial of supervised treatment of psoriasis vulgaris in a day‐care setting. British Journal of Dermatology 2006; Vol. 155, issue 4:800‐7. [PUBMED: 16965431] - PubMed
Van de Kerkhof 2009 {published and unpublished data}
-
- Kerkhof P, Anstey A, Barnes L, Bolduc C. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients in the same vehicle [Abstract P2734.] American Academy of Dermatology 65th Annual Meeting 2‐6 February, 2007. Journal of the American Academy of Dermatology 2007;56(2):AB182.
-
- Kerkhof PCM, Hoffmann V, Anstey A, Barnes L, Bolduc C, Reich K, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. British Journal of Dermatology 2009; Vol. 160, issue 1:170‐6. [PUBMED: 18694678] - PubMed
Vanderploeg 1976 {published data only}
-
- Vanderploeg DE. Betamethasone Dipropionate Ointment in the Treatment of Psoriasis and Atopic Dermatitis: A Double‐Blind Study. Southern Medical Journal 1976;69(7):862‐3. [PUBMED: 781848] - PubMed
Van der Vleuten 1995 {published data only}
-
- Vleuten CJM, Jong EMGJ, Rulo EHFC, Gerritsen MJP, Kerkhof PCM. In‐Patient Treatment with Calcipotriol Versus Dithranol in Refractory Psoriasis. European Journal of Dermatology 1995;5(8):676‐9. [EMBASE: 1996010645]
Veien 1997 {published and unpublished data}
-
- Veien NK, Bjerke JR, Rossmann‐Ringdahl I, Jakobsen HB. Once daily treatment of psoriasis with tacalcitol compared with twice daily treatment with calcipotriol. A double‐blind trial. British Journal Of Dermatology 1997;137(4):581‐6. [PUBMED: 9390335] - PubMed
Vladimirov 1994 {published data only}
-
- Vladimirov VV, Tcherjomukchina IJ, Kurjanova ON, Menshikova LV, Mazina NM. Efficacy of calcipotriol ointment compared to betamethasone 17‐valerate ointment in the treatment of psoriasis [Abstract]. Skin Therapy Update '94. Crete, Greece, 1994:219.
Volden 1992 {published data only}
-
- Volden G, Bjornberg A, Tegner E, Pedersen NB, Arles UB, Agren S, et al. Short‐Contact Treatment at Home with Micanol. Acta Dermato Venereologica 1992;172(Suppl):20‐2. [PUBMED: 1585757] - PubMed
Wall 1998 {published and unpublished data}
-
- Wall AR, Poyner TF. Psoriasis; the Burden of Disease Before, During and After Treatment with Dovonex Ointment or Dithrocream. British Journal Of Dermatology 1997;137(Suppl 50):55.
-
- Wall ARJ, Poyner TF, Menday AP. A comparison of treatment with dithranol and calcipotriol on the clinical severity and quality of life in patients with psoriasis. British Journal Of Dermatology 1998;139(6):1005‐11. [PUBMED: 9990363] - PubMed
Weinstein 1996 {published data only}
-
- Weinstein GD. Safety, Efficacy and Duration of Therapeutic Effect of Tazarotene Used in the Treatment of Plaque Psoriasis. British Journal Of Dermatology 1996;135(Suppl 49):32‐6. [PUBMED: 9035703] - PubMed
-
- Weinstein GD, Krueger GG, Lowe NJ, Duvic M, Friedman DJ, Jegasothy BV, et al. Tazarotene gel, a new retinoid, for topical therapy of psoriasis: vehicle‐controlled study of safety, efficacy, and duration of therapeutic effect. Journal of the American Academy of Dermatology 1997;37(1):85‐92. [PUBMED: 9216528] - PubMed
Weinstein 2003 {published data only}
-
- Weinstein GD. Tazarotene cream in the treatment of plaque psoriasis [Abstract]. 20th World Congress of Dermatology, 1‐5 July 2002, Paris. 2002:P2043.
-
- Weinstein GD, Koo JY, Krueger GG, Lebwohl MG, Lowe NJ, Menter MA, et al. Tazarotene cream in the treatment of psoriasis: Two multicenter, double‐blind, randomized, vehicle‐controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. Journal of the American Academy of Dermatology 2003;48(5):760‐7. [PUBMED: 12734506] - PubMed
White 2006 (H) {published data only}
-
- White S, Vender R, Thaci D, Haverkamp C, Naeyaert J‐M, Foster R, et al. Use of calcipotriene cream (Dovonex cream) following acute treatment of psoriasis vulgaris with the calcipotriene/betamethasone dipropionate two‐compound product (Taclonex): a randomized, parallel‐group clinical trial. American Journal of Clinical Dermatology 2006; Vol. 7, issue 3:177‐84. [PUBMED: 16734505] - PubMed
White 2006 (P) {published data only}
-
- White S, Vender R, Thaci D, Haverkamp C, Naeyaert J‐M, Foster R, et al. Use of calcipotriene cream (Dovonex cream) following acute treatment of psoriasis vulgaris with the calcipotriene/betamethasone dipropionate two‐compound product (Taclonex): a randomized, parallel‐group clinical trial. American Journal of Clinical Dermatology 2006; Vol. 7, issue 3:177‐84. [PUBMED: 16734505] - PubMed
Wolska 1995 {published data only}
-
- Wolska H, Laws S, Schulz‐Kiesow M, Grossman R, Jablonska S, Stadlers R, et al. Clinical evaluation of a PAF‐antagonist in psoriasis vulgaris. Journal Of Dermatological Treatment 1995;6(1):43‐5. [EMBASE: 1995125049]
Wortzel 1975 (1) {published data only}
-
- Wortzel MH. A new corticosteroid for moderate/severe dermatoses. Clinical Medicine 1975;82(3):23‐6.
Wortzel 1975 (2) {published data only}
-
- Wortzel WH. A new corticosteroid for moderate/severe dermatoses. Clinical Medicine 1975;82(3):23‐6.
Wozel 2001 {published and unpublished data}
-
- Wozel G. Treatment of chronic‐stationary psoriasis with fluocinolone acetonide and calcipotriol. Zeitschrift Fur Hautkrankheiten 2001;76(7‐8):482.
Yang 2009 {published data only}
-
- Yang DQ, Zhang LX, Bai YP. Clinical observation of sequential therapy of calcipotriol cream with halometasone cream in plaque type psoriasis. Journal of Clinical Dermatology 2009;38(6):401‐3. [EMBASE: 2010312999]
Zonneveld 1998 (H) {published data only}
-
- Zonneveld IM, Rubins A, Jablonska S, Dobozy A, Ruzicka T, Kind P, et al. Topical tacrolimus is not effective in chronic plaque psoriasis. A pilot study. Archives of Dermatology 1998;134(9):1101‐2. [PUBMED: 9762021] - PubMed
Zonneveld 1998 (P) {published data only}
-
- Zonneveld IM, Rubins A, Jablonska S, Dobozy A, Ruzicka T, Kind P, et al. Topical tacrolimus is not effective in chronic plaque psoriasis. A pilot study. Archives of Dermatology 1998;134(9):1101‐2. [PUBMED: 9762021] - PubMed
References to studies excluded from this review
Agrawal 2010 {published data only}
-
- Agrawal Y, Petkar KC, Sawant KK. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. International Journal of Pharmaceutics 2010; Vol. 401, issue 1‐2:93‐102. [0378‐5173] - PubMed
Akerman 2009 {published data only (unpublished sought but not used)}
-
- Akerman L, Sredni B, Albeck M, David M. Topical Ammonium‐trichlorotellurate (AS101) in the treatment of mild plaque‐type psoriasis; double‐blind placebo controlled study. Journal of Investigative Dermatology 2009;129(Suppl. 1):S50.
Ambroziak 2002 {published data only}
-
- Ambroziak M, Stapor W, Langner A, Kwiek B. Clinical evaluation of the scalp psoriasis treatment with clobetasol propionate 0.05% lotion [Ocena skutecznosci I bezpieczenstwa stosowania plynu zawierajacego 0,05% propionianu klobetazolu w leczeniu przewleklej luszczycy plackowatej skory owlosionej glowy]. Przeglad Dermatologiczny 2002;89(3):237‐40.
Baadsgaard 1995 {published data only}
-
- Baadsgaard O, Traulsen J, Roed Petersen J, Jakobsen HB. Optimal concentration of tacalcitol in once‐daily treatment of psoriasis. Journal Of Dermatological Treatment 1995;6(3):145‐50.
Baran 1999 {published data only}
-
- Baran R, Tosti A. Topical treatment of nail psoriasis with a new corticoid‐containing nail lacquer formulation. Journal Of Dermatological Treatment 1999;10:201‐4.
Bianchi 2003 {published data only}
-
- Bianchi L, Soda R, Diluvio L, Chimenti S. Tazarotene 0.1% gel for psoriasis of the fingernails and toenails: an open, prospective study. British Journal of Dermatology 2003;149(1):207‐9. - PubMed
Brodell 2011a {published data only (unpublished sought but not used)}
-
- Brodell R, Goffe BS, Weiss JS, Bruce S. Safety and efficacy of two regimens involving clobetasol spray, 0.05% and calcitriol ointment, 3 mug/g for moderate plaque psoriasis. Journal of the American Academy of Dermatology 2011; Vol. 64, issue 2 Suppl 1:AB149.
Buder 2010 {published data only}
-
- Buder K, Knuschke P, Wozel G. Evaluation of methylprednisolone aceponate, tacrolimus and combination thereof in the psoriasis plaque test using sum score, 20‐MHz‐ultrasonography and optical coherence tomography. International Journal of Clinical Pharmacology & Therapeutics 2010;48(12):814‐20. - PubMed
Callen 1996 {published data only}
-
- Callen J. Comparison of Safety and Efficacy of Fluticasone Propionate Cream, 0.05%, and Betamethasone Valerate Cream, 0.1%, in the Treatment of Moderate‐to‐Severe Psoriasis. Cutis 1996;57(2 Suppl):45‐50. - PubMed
Cannavo 2003 {published and unpublished data}
-
- Cannavo SP, Guarneri F, Vaccaro M, Borgia F, Guarneri B. Treatment of psoriatic nails with topical cyclosporin: a prospective, randomized placebo‐controlled study. Dermatology 2003; Vol. 206, issue 2:153‐6. [MEDLINE: ] - PubMed
-
- Cannavo SP, Guarneri F, Vaccaro M, Borgia FAD, Institute of Dermatology University of Messina Italy. Treatment of psoriatic nails with topical cyclosporin. 11th Congress of the European Academy of Dermatology and Venereology. 2002:P27‐89.
Carroll 2005 {published data only}
-
- Carroll CL, Clarke J, Camacho F, Balkrishnan R, Feldman SR. Topical tacrolimus ointment combined with 6% salicylic acid gel for plaque psoriasis treatment. Archives of Dermatology 2005;141(1):43‐6. - PubMed
De Jong 1999 {published and unpublished data}
-
- Jong EM, Menke HE, Praag MC, Kerkhof PC. Dystrophic psoriatic fingernails treated with 1% 5‐fluorouracil in a nail penetration‐enhancing vehicle: a double‐blind study. Dermatology 1999;199(4):313‐8. - PubMed
Elias 1994 {published data only}
-
- Elias AN, Dangaran K, Barr RJ, Rohan MK, Goodman MM. A controlled trial of topical propylthiouracil in the treatment of patients with psoriasis. Journal of the American Academy of Dermatology 1994;31(3 Pt 1):455‐8. - PubMed
Esposito 2009 {published data only}
-
- Esposito M. The therapeutic advantages on BMV plaster in chronic plaque psoriasis. Results from a new European multicentric study [Les avantages therapeutiques d'un topique bio‐adhesif a base de VBM dans le traitement du psoriasis en plaques. Presentation des resultats d'une nouvelle etude europeenne, multicentrique]. Annales de Dermatologie et de Venereologie 2009; Vol. 136, issue Suppl 8:S476‐80. [0151‐9638]
Friedrich 2004 {published data only}
-
- Friedrich M, Vollhardt K, Zahlten R, Sterry W, Wolff G. Demonstration of antipsoriatic efficacy of a new topical formulation of the small molecule selectin antagonist bimosiamose [Abstract P016]. European Congress on Psoriasis 2004. Journal of the European Academy of Dermatology & Venereology 2004; Vol. 18, issue 6:779.
Hsia 2010 {published data only}
-
- Hsia E, Hofland H, Therrien J‐P, Chern W. In vitro activity and safety assessment of a novel calcipotriene foam formulation. 68th Annual Meeting of the American Academy of Dermatology, Miami, FL United States. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB138.
Insa 2009 {published data only}
-
- Insa R, Cruz G, Gassmueller J, Leon M. Topical cyclosporin proves efficacy in psoriatic patients in a phase I/II clinical trial. 67th Annual Meeting of the American Academy of Dermatology, San Francisco, CA United States. Journal of the American Academy of Dermatology 2009; Vol. 60, issue 3 Suppl 1:AB180. [0190‐9622]
Iraji 2010 {published data only}
-
- Iraji F, Faghihi G, Siadat AH, Enshaieh S, Shahmoradi Z, Joia A, et al. Efficacy of 15% azelaic acid in psoriasis vulgaris: a randomized, controlled clinical trial. Journal of Drugs in Dermatology 2010;9(8):964‐8. - PubMed
Ito 2005 {published data only}
-
- Ito Y, Nakagawa H. A comparative study of maxacalcitol monotherapy, clobetasol propionate monotherapy and the combination of each agent as a premixed ointment for the treatment of psoriasis. [Japanese]. Nishinihon Journal of Dermatology 2005;67(5):522‐6.
Jansen 1986 {published data only}
-
- Jansen C, Lammintausta K, Bullingham RES, Forsstrom S. A Clinical Trial of Lonapalene Fluocinolone Acetonide and Vehicle in Psoriasis [Abstract]. Journal of Investigative Dermatology 1986;86(4):483.
Kaur 2004 {published data only}
-
- Kaur I, Jain R, Kumar B. Comparative study of calcipotriol (0.005%) vs tazarotene (0.05%, 0.1%) in stable plaque psoriasis [Abstract P015]. European Congress on Psoriasis 2004. Journal of the European Academy of Dermatology & Venereology 2004; Vol. 18, issue 6:779.
Kleyn 2005 {published data only}
-
- Kleyn CE, Woodcock D, Sharpe GR. The efficacy of 0.1% tacrolimus ointment compared with clobetasone butyrate 0.05% ointment in patients with facial, flexural or genital psoriasis [Abstract P‐41]. The 85th BAD Annual Meeting 5‐8th July 2005, Glasgow, UK. British Journal of Dermatology 2005;153(Suppl 1):33.
Kragballe 1989 {published data only}
-
- Kragballe K. Treatment of psoriasis by the topical application of the novel cholecalciferol analogue calcipotriol (MC 903). Archives of Dermatology 1989;125(12):1647‐52. - PubMed
Kragballe 1994 {published data only}
-
- Kragballe K, Dam TN, Hansen ER, Baadsgaard O, Gronhoj Larsen F, Sondergaard J, et al. Efficacy and Safety of the 20‐Epi‐Vitamin D3 Analogue Kh 1060 in the Topical Therapy of Psoriasis: Results of a Dose‐Ranging Study. Acta Dermato‐Venereologica 1994;74(5):398‐402. - PubMed
Lebwohl 1998b {published data only}
-
- Lebwohl M, Yoles A, Lombardi K, Lou W. Calcipotriene Ointment and Halobetasol Ointment in the Long‐Term Treatment of Psoriasis: Effects on the Duration of Improvement. Journal of the American Academy of Dermatology 1998;39(3):447‐50. - PubMed
Lebwohl 2001 {published data only}
-
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. International Journal Of Dermatology 2001;40(1):64‐6. [MEDLINE: ] - PubMed
Levin 2003 {published data only}
-
- Levin C, Fiorentino DF, Vosganian G, Chon S, Kimball AB. The safety of topical cyclosporin A conjugate (CGC1072) in the treatment of mild to moderate psoriasis [Abstract 1201] International Investigative Dermatology. The 4th Joint Meeting of the ESDR, Japanese SID & SID, 30th April‐4th May 2003, Florida, USA. Journal of Investigative Dermatology 2003;121(1):201.
Meyrat 1996 {published and unpublished data}
-
- Meyrat R, Muller I. Daivonex registered trade mark ointment twice a day versus Daivonex registered trade mark cream in the morning and Daivonex registered trade mark ointment in the evening [Daivonex Salbe zwei mal taglich versus Daivonex Creme morgens und Daivonex Salbe abends]. Ars Medici 1996;86(20):1218‐20.
Palazon 2005 {published data only}
-
- Palazon DAB, Sanchez‐Regana M, Mateos FL, Ezquerra GM, Acosta EH, Vidal I, et al. A double blind aleatory and prospective study of topical vitamin b12 in psoriasis [Abstract P06.21]. The 14th Congress of the European Academy of Dermatology and Venereology, London, UK. 12‐15th October 2005. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):165.
Reygagne 2002a {published data only}
-
- Reygagne P, Diaconu J, Près H, Ernst TM, Meyer KG, S.A.D.A. Efficacy and safety comparison of clobetasol propionate shampoo, gel and vehicle in scalp psoriasis. 11th Congress of the European Academy of Dermatology and Venereology. 2002:P27‐9.
Rhemus 2006 {published data only}
-
- Rhemus W, Kim J, Kern D, Murase J. A double‐blind, placebo‐controlled study of a natural topical anti‐inflammatory for treatment of atopic dermatitis and psoriasis [Abstract P2849]. American Academy of Dermatology 64th Annual Meeting March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB210.
Ruzicka 2004 {published data only}
-
- Ruzicka T, Trompke C. Treatment of scalp psoriasis. An effective and safe tacalcitol emulsion [Behandlung der kopfhaut‐psoriasis. Gute wirksamkeit und sicherheit durch Tacalcitol‐emulsion]. Hautarzt 2004;55(2):165‐70. - PubMed
Sander 1998 {published data only}
-
- Sander P, Stucker M, Hermes N, Hoffmann K, Altmeyer P. Mometasone and calcipotriol optimise the initial therapeutic effect of dithranol on chronic plaque psoriasis [Mometason Und Calcipotriol Optimieren Den Therapeutischen Initialeffekt Von Dithranol Auf Die Chronisch Stationare Psoriasis (CSP)]. Hautarzt 1998;49(4):291‐4. - PubMed
Saraswat 2007 {published data only}
-
- Saraswat A, Agarwal R, Katare OP, Kaur I, Kumar B. A randomized, double‐blind, vehicle‐controlled study of a novel liposomal dithranol formulation in psoriasis. Journal of Dermatological Treatment 2007; Vol. 18, issue 1:40‐5. - PubMed
Scher 2001 {published data only}
-
- Scher R. Tazarotene 0.1% gel in the treatment of nail psoriasis [Abstract]. 20th World Congress of Dermatology, 1st to 5th July 2002. 2002:P0791.
-
- Scher RK, Stiller M, Zhu YI. Tazarotene 0.1% gel in the treatment of fingernail psoriasis: a double‐blind, randomized, vehicle‐controlled study. Cutis 2001;68(5):355‐8. [PUBMED: 11766122 ] - PubMed
-
- Scher RK, Stiller M, Zhu YI. Treating fingernail psoriasis with tazarotene 0.1% gel: A vehicle‐controlled study [Abstract]. 2nd Joint Meeting International Psoriasis Symposium and European Congress on Psoriasis. Skin Pharmacology & Applied Skin Physiology 2001;14(3):170.
Sefton 1984 {published data only}
-
- Sefton J, Loder JS, Kyriakopoulos AA. Clinical Evaluation of Hydrocortisone Valerate 0.2% Ointment. Clinical Therapeutics 1984;6(3):282‐93. - PubMed
Sharma 2003 {published data only}
-
- Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: a prospective randomized study in stable plaque psoriasis. International Journal of Dermatology 2003;42(10):834‐8. - PubMed
Syed 2001a {published data only}
-
- Syed TA, Qureshi ZA. Management of psoriasis of the scalp with methotrexate (0.25%) in an aerosolized spray gel. A placebo‐controlled, double‐blind study [Abstract]. 2nd Joint Meeting International Psoriasis Symposium and European Congress on Psoriasis. Skin Pharmacology & Applied Skin Physiology 2001;14(3):167.
Tokura 2004 {published data only}
-
- Tokura Y. Effectiveness of Combination between Diflucortolone Valerate and Vitamin D3 Analogue in the Treatment of Psoriasis Vulgaris. [Japanese]. Nishinihon Journal of Dermatology 2004;66(2):188‐191.
Tosti 1998 {published data only}
-
- Tosti A, Piraccini BM, Cameli N, Kokely F, Plozzer C, Cannata GE, et al. Calcipotriol Ointment in Nail Psoriasis: A Controlled Double‐Blind Comparison with Betamethasone Dipropionate and Salicylic Acid. British Journal Of Dermatology 1998;139(4):655‐9. [MEDLINE: ] - PubMed
Tzaneva 2003 {published data only}
-
- Tzaneva S, Honigsmann H, Tanew A. Observer‐blind, randomized, intrapatient comparison of a novel 1% coal tar preparation (Exorex) and calcipotriol cream in the treatment of plaque type psoriasis. British Journal of Dermatology 2003;149(2):350‐3. [EMBASE: 2003371589] - PubMed
van de Kerkhof 1996b {published data only}
-
- Kerkhof PCM, Kuypers A. A randomised, double blind, left right study to compare hydrocortisone 17‐butyrate 0.1% emulsion with vehicle in the treatment of patients suffering from psoriasis vulgaris [Poster P16]. 6th International Skin Symposium. Brussels, 1996.
Vena 2005 {published data only}
-
- Vena GA, Cassano N, Agnusdei CP, Bellini M, Calabretta S, Centofanti S, et al. Treatment of psoriasis vulgaris with calcipotriol betamethasone dipropionate combination followed by calcipotriol and assessment of the adjuvant basic use of urea‐based emollients. European Journal of Inflammation 2005; Vol. 3, issue 1:37‐41.
References to studies awaiting assessment
Bissonnette 2011 {published data only}
-
- Bissonnette R, Bolduc C, Maari C, Nigen S, Webster JM, Tang L, et al. Efficacy and safety of topical WBI‐1001 in patients with mild to moderate psoriasis: results from a randomized double‐blind placebo‐controlled, phase II trial. Journal of the European Academy of Dermatology & Venereology 2012;26(12):1516‐21. [DOI: 10.1111/j.1468-3083.2011.04332.x; NCT01098721; PUBMED: 22077962] - DOI - PubMed
-
- NCT01098721. A Safety/Efficacy Study of a Non‐steroid, Topical Cream Treatment of Psoriasis Over 12‐weeks (134993). clinicaltrials.gov/ct2/show/NCT01098721 (accessed January 2013).
Callis Duffin 2010 {published data only}
-
- Callis Duffin K, Luchi M, Fidelus‐Gort R, Newton R, Fridman J, Burn T, et al. Novel mechanism for topical treatment of plaque psoriasis – results of a randomized, double blind, concentration ranging, vehicle controlled 12 week study with JAK 1/2 inhibitor INCB018424 cream (Abstract 261). 2010 Annual Meeting of the Society for Investigative Dermatology, Hilton Atlanta, Atlanta, Georgia, May 5‐8. Journal of Investigative Dermatology 2010;130(Suppl 1):S44.
-
- NCT00778700. A Dose Ranging Study of the Effect of INCB018424 Phosphate Cream When Applied to Patients With Plaque Psoriasis. clinicaltrials.gov/show/NCT00778700 (accessed January 2013).
Calzavara‐Pinton 2011 {published data only}
-
- Calzavara‐Pinton P, Rossi M T, Sala R, Venturini M. The separate daily application of tacalcitol 4 microg/g ointment and budesonide 0.25 mg/g cream is more effective than the single daily application of a two compound ointment containing calcipotriol 50 microg/g and betamethasone dipropionate 0.5 mg/g. Giornale Italiano di Dermatologia e Venereologia 2011;146(4):295‐9. - PubMed
Henry 2011 {published data only}
-
- Henry M, Frankel A, Emer J, Lebwohl M. Bilateral comparison study on the order of application of combination clobetasol proprionate spray and calcitriol ointment in the treatment of plaque psoriasis. (Abstract P3343). Conference: 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Conference Publication. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB156.
Katoh 2003 {published data only}
-
- Katoh N, Kishimoto S. Combination of calcipotriol and clobetasol propionate as a premixed ointment for the treatment of psoriasis. European Journal of Dermatology 2003;13(4):382‐4. [MEDLINE: ] - PubMed
Matheson 2011 {published data only}
-
- Matheson R, Hsia E, Ge X. Safety and bioavailability of two calcipotriene formulations in subjects with mild to moderate plaque‐type psoriasis. (Abstract P3348). Conference: 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB157.
Paulsen 2005 {published data only}
-
- Paulsen E, Korsholm L, Brandrup F. A double‐blind, placebo‐controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. Journal Of The European Academy Of Dermatology & Venereology 2005;19(3):326‐31. [MEDLINE: ] - PubMed
Sadeghian 2011 {published data only}
-
- Sadeghian G, Ziaei H, Nilforoushzadeh M A. Treatment of localized psoriasis with a topical formulation of zinc pyrithione. Acta Dermatovenerologica Alpina, Panonica et Adriatica 2011;20(4):187‐90. - PubMed
Vahlquist 2004 {published data only}
-
- Vahlquist A, Torma H, Carlsson B. Inefficacy of topical thyroid hormone analogue TriAc in plaque psoriasis: results of a double‐blind placebo‐controlled trial. British Journal of Dermatology 2004;151(2):489‐91. [MEDLINE: ] - PubMed
Yang 1999 {published data only}
-
- Yang XY, Lin L, Cui PG, Jia H, Jin PY, Liu XQ. Comparison of the efficacy of tacalcitol ointment and 17 a hydrocortisone cream in the treatment of psoriasis (Chinese). Chinese Journal of Dermatology 1999;32(6):425.
References to ongoing studies
NCT00824980 {unpublished data only}
-
- NCT00824980. Combined Inhibition of Dipeptidyl Peptidase IV (DPIV/CD26) and Aminopeptidase N (APN/CD13) in the Treatment of Psoriasis. clinicaltrials.gov/ct2/show/NCT00824980 (accessed December 2011).
NCT01018134 {unpublished data only}
-
- NCT01018134. Desoximetasone Spray 0.05%, 0.25%; Dose Ranging Study. clinicaltrials.gov/ct2/show/NCT01018134 (accessed December 2011).
NCT01206387 {unpublished data only}
-
- NCT01206387 . Effects of Desoximetasone Spray 0.25% on Moderate to Severe Plaque Psoriasis. clinicaltrials.gov/ct2/show/NCT01206387 (accessed December 2011).
NCT01206660 {unpublished data only}
-
- NCT01206660. Effects of Desoximetasone Spray 0.25% in Patients With Moderate to Severe Plaque Psoriasis. clinicaltrials.gov/ct2/show/NCT01206660 (accessed December 2011).
NCT01246583 {unpublished data only}
-
- NCT01246583. CP‐690‐550 Ointment For Chronic Plaque Psoriasis. clinicaltrials.gov/ct2/show/NCT01246583 (accessed December 2011).
NCT01247818 {unpublished data only}
-
- NCT01247818 . Randomized Study of PH‐10 for Psoriasis. clinicaltrials.gov/ct2/show/NCT01247818 (accessed December 2011).
NCT01422434 {unpublished data only}
-
- NCT01422434. LEO 90105 Ointment in Japanese Subjects With Psoriasis. clinicaltrials.gov/ct2/show/results/NCT01422434 (accessed December 2011).
NCT01465282 {unpublished data only}
-
- NCT01465282. Evaluation of the Efficacy, Safety and Toleration of CT327 Ointment in Patients With Psoriasis Vulgaris. clinicaltrials.gov/ct2/show/NCT01465282 (accessed December 2011).
NCT01536886 {unpublished data only}
-
- NCT01536886. LEO 90100 Compared With Calcipotriol Plus Betamethasone Dipropionate Ointment, LEO 90100 Vehicle and Ointment Vehicle in Subjects With Psoriasis Vulgaris. clinicaltrials.gov/show/NCT01536886 (accessed August 2012).
NCT01536938 {unpublished data only}
-
- NCT01536938. LEO 90100 in the Treatment of Psoriasis Vulgaris. clinicaltrials.gov/show/NCT01536938 (accessed August 2012).
Additional references
Afifi 2005
Andres 2006
-
- Andres P, Poncet M, Farzaneh S, Soto P. Short‐term safety assessment of clobetasol propionate 0.05% shampoo: hypothalamic‐pituitary‐adrenal axis suppression, atrophogenicity, and ocular safety in subjects with scalp psoriasis. Journal of Drugs in Dermatology 2006; Vol. 5, issue 4:328‐32. [MEDLINE: ] - PubMed
Ashcroft 2000
-
- Ashcroft DM, Li Wan Po A, Williams HC, Griffiths CE. Cost‐effectiveness analysis of topical calcipotriol versus short‐contact dithranol in the treatment of mild to moderate plaque psoriasis. Pharmacoeconomics 2000;18(5):469‐76. [MEDLINE: ] - PubMed
Ashcroft 2000a
Aste 2004
-
- Aste N. Tacalcitol ointment in the treatment of psoriasis [Tacalcitolo unguento nel trattamento della psoriasi]. Giornale Italiano di Dermatologia e Venereologia 2004;139(1):81‐4. [EMBASE: 2004234976]
Atkinson 2004
-
- Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health & Quality of Life Outcomes 2004;2(1):12. [MEDLINE: ] - PMC - PubMed
Balkrishnan 2003
-
- Balkrishnan R, Carroll CL, Camacho FT, Feldman SR. Electronic monitoring of medication adherence in skin disease: Results of a pilot study. Journal of the American Academy of Dermatology 2003;49(4):651‐4. [MEDLINE: ] - PubMed
Barisic‐Drusko 1994
-
- Barisic‐Drusko V, Dobric I, Pasic A, Paljan D, Jukic Z, Basta‐Juzbasic A, et al. Frequency of psoriatic arthritis in general population and among the psoriatics in department of dermatology. Acta Dermato‐Venereologica. Supplementum. 1994;186:107‐8. [MEDLINE: ] - PubMed
Barker 1991
-
- Barker JN. The pathophysiology of psoriasis. Lancet 1991;338(8761):227‐30. [MEDLINE: ] - PubMed
Barnes 2000
-
- Barnes L, Altmeyer P, Forstrom L, Stenstrom MH. Long‐term treatment of psoriasis with calcipotriol scalp solution and cream. European Journal of Dermatology 2000;10(3):199‐204. [MEDLINE: ] - PubMed
Berth‐Jones 1992c
-
- Berth‐Jones J. Immediate and long term effects of topical calcipotriol on calcium homeostasis during treatment of psoriasis [Abstract]. British Journal of Dermatology 1992;127(Suppl 40):17.
Berth‐Jones 1993
-
- Berth‐Jones J, Bourke JF, Iqbal SJ, Hutchinson PE. Urine Calcium Excretion During Treatment of Psoriasis with Topical Calcipotriol. British Journal Of Dermatology 1993;129(4):411‐4. [MEDLINE: ] - PubMed
Bhalerao 1998
-
- Bhalerao J, Bowcock AM. The genetics of psoriasis: a complex disorder of the skin and immune system. Human Molecular Genetics 1998;7(10):1537‐45. [MEDLINE: ] - PubMed
Bleiker 1998
-
- Bleiker TO, Bourke JF, Mumford R, Hutchinson PE. Long‐Term Outcome of Severe Chronic Plaque Psoriasis Following Treatment with High‐Dose Topical Calcipotriol. British Journal Of Dermatology 1998;139(2):285‐6. [MEDLINE: ] - PubMed
BMA 2012
-
- Joint Formulary Committee. British National Formulary (BNF) 63. London: Pharmaceutical Press, 2012.
Bonifati 1998
-
- Bonifati C, Carducci M, Mussi A, D'Auria L, Ameglio F. Recognition and treatment of psoriasis: special considerations in elderly patients. Drugs & Aging 1998;12(3):177‐90. [MEDLINE: ] - PubMed
Bos 2002
-
- Bos JD. Topical tacrolimus and pimecrolimus are not associated with skin atrophy. British Journal of Dermatology 2002;146(2):342. [MEDLINE: ] - PubMed
Bos 2008
-
- Bos JD, Spuls PI. Topical treatments in psoriasis: today and tomorrow. Clinics in Dermatology 2008; Vol. 26, issue 5:432‐7. [MEDLINE: ] - PubMed
Bourke 1993a
-
- Bourke JF, Berth‐Jones J, Iqbal SJ, Hutchinson PE. High‐dose topical calcipotriol in the treatment of extensive psoriasis vulgaris. British Journal of Dermatology 1993;129(1):74‐6. [MEDLINE: ] - PubMed
Bourke 1994
-
- Bourke JF, Berth‐Jones J, Mumford R, Iqbal SJ, Hutchinson PE. High dose topical calcipotriol consistently reduces serum parathyroid hormone levels. Clinical Endocrinology 1994;41(3):295‐7. [MEDLINE: ] - PubMed
Brandrup 1978
-
- Brandrup F, Hauge M, Henningsen K, Eriksen B. Psoriasis in an unselected series of twins. Archives of Dermatology 1978;114(6):874‐8. [MEDLINE: ] - PubMed
Breneman 2007
-
- Breneman D, Sheth P, Berger V, Naini V, Stevens V. Phase II clinical trial of bexarotene gel 1% in psoriasis. Journal of Drugs in Dermatology 2007; Vol. 6, issue 5:501‐6. [MEDLINE: ; 1545‐9616] - PubMed
Brodell 2011b
-
- Brodell RT, Bruce S, Hudson CP, Weiss JS, Colon LE, Johnson LA, et al. A multi‐center, open‐label study to evaluate the safety and efficacy of a sequential treatment regimen of clobetasol propionate 0.05% spray followed by Calcitriol 3 mg/g ointment in the management of plaque psoriasis. Journal of Drugs in Dermatology 2011; Vol. 10, issue 2:158‐64. [MEDLINE: ; 1545‐9616] - PubMed
Brownell 2007
-
- Brownell I, Strober BE. Folate with methotrexate: big benefit, questionable cost. British Journal of Dermatology 2007; Vol. 157, issue 1:213. [MEDLINE: ] - PubMed
Bruner 2003a
-
- Bruner CR, Feldman SR, Ventrapragada M, Fleischer AB Jr. A systematic review of adverse effects associated with topical treatments for psoriasis. Dermatology Online Journal 2003; Vol. 9, issue 1:2. [MEDLINE: ] - PubMed
Camp 1992
-
- Camp RDR. Psoriasis. In: Champion RH, Burton JL, Ebling FJG editor(s). Textbook of Dermatology. Oxford: Blackwell Science, 1992:1391‐458.
Carboni 2005
-
- Carboni I, Felice C, Bergamin A, Chimenti S. Topical use of calcitriol 3 microg/g ointment in the treatment of mild‐to‐moderate psoriasis: results from an open‐label study. Journal of the European Academy of Dermatology & Venereology 2005; Vol. 19, issue Suppl 3:11‐3. [MEDLINE: ; 0926‐9959] - PubMed
Carey 2006
-
- Carey W, Glazer S, Gottlieb AB, Lebwohl M, Leonardi C, Menter A, et al. Relapse, rebound, and psoriasis adverse events: an advisory group report. Journal of the American Academy of Dermatology 2006; Vol. 54, issue 4 Suppl 1:S171‐81. [MEDLINE: ] - PubMed
Carroll 2004a
-
- Carroll CL, Feldman SR, Camacho FT, Balkrishnan R. Better medication adherence results in greater improvement in severity of psoriasis. British Journal of Dermatology 2004;151(4):895‐7. [MEDLINE: ] - PubMed
Carroll 2004b
-
- Carroll CL, Feldman SR, Camacho FT, Manuel JC, Balkrishnan R. Adherence to topical therapy decreases during the course of an 8‐week psoriasis clinical trial: commonly used methods of measuring adherence to topical therapy overestimate actual use. Journal of the American Academy of Dermatology 2004;51(2):212‐6. [MEDLINE: ] - PubMed
Chalmers 2000
Chalmers 2006
Chen 2011
Chu 2000
-
- Chu T. Better patient compliance in psoriasis. Practitioner 2000;244(1608):238‐42. [MEDLINE: ] - PubMed
Corbett 1976
-
- Corbett MF. The response of psoriasis to betamethasone valerate and clobetasol propionate: a 6‐month controlled study. British Journal Of Dermatology 1976;94(Suppl 12):89‐93. [MEDLINE: ] - PubMed
Cork 2003
-
- Cork MJ, Britton J, Butler L, Young S, Murphy R, Keohane SG. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. British Journal of Dermatology 2003;149(3):582‐9. [MEDLINE: ] - PubMed
Cossmann 2006
-
- Cossmann M, Welzel J. Evaluation of the atrophogenic potential of different glucocorticoids using optical coherence tomography, 20‐MHz ultrasound and profilometry; a double‐blind, placebo‐controlled trial. British Journal of Dermatology 2006; Vol. 155, issue 4:700‐6. [PUBMED: 16965418] - PubMed
Cullen 1996
-
- Cullen SI. Long‐Term Effectiveness and Safety of Topical Calcipotriene for Psoriasis. Calcipotriene Study Group. Southern Medical Journal 1996;89(11):1053‐6. [MEDLINE: ] - PubMed
de Jong 1997
-
- Jong EM. The course of psoriasis. Clinics in Dermatology 1997;15(5):687‐92. [MEDLINE: ] - PubMed
de Tiedra 1997
-
- Tiedra A, Mercadal J, Lozano R. Prednicarbate versus fluocortin for inflammatory dermatoses. A cost‐effectiveness study. Pharmacoeconomics 1997;12(2 Pt 1):193‐208. [MEDLINE: ] - PubMed
de Vries 2013
du Vivier 1975
-
- du Vivier A, Stoughton RB. Tachyphylaxis to the action of topically applied corticosteroids. Archives of Dermatology 1975;111(5):581‐3. [MEDLINE: ] - PubMed
Farber 1974
-
- Farber EM, Nall ML, Watson W. Natural history of psoriasis in 61 twin pairs. Archives of Dermatology 1974;109(2):207‐11. [MEDLINE: ] - PubMed
Feldman 1997
-
- Feldman SR, Fleischer AB, Reboussin DM, Rapp SR, Bradham DD, Exum ML, et al. The economic impact of psoriasis increases with psoriasis severity. Journal of the American Academy of Dermatology 1997;37(4):564‐9. [MEDLINE: ] - PubMed
Feldman 2000
-
- Feldman SR, Sahu S, Fleischer AB, Dezii CM. Per‐gram cost of medication is by itself a poor indicator for comparing costs of different psoriasis treatments: a retrospective cohort study of the cost of psoriasis treatment with topical corticosteroids versus topical calcipotriene. Journal of Cutaneous Medicine & Surgery 2000;4(3):121‐5. [MEDLINE: ] - PubMed
Feldman 2007
-
- Feldman SR, Camacho FT, Krejci‐Manwaring J, Carroll CL, Balkrishnan R. Adherence to topical therapy increases around the time of office visits. Journal of the American Academy of Dermatology 2007;57(1):81‐3. [MEDLINE: ] - PubMed
Feldman 2007a
-
- Feldman SR. Effectiveness of clobetasol propionate spray 0.05% added to other stable treatments: add‐on therapy in the COBRA trial. Cutis 2007; Vol. 80, issue 5 Suppl:20‐8. [MEDLINE: ; 0011‐4162] - PubMed
Ferrandiz 1998
-
- Ferrandiz C, Bielsa I, Ribera M, Fuente MJ, Carrascosa JM. Reinforcement Therapy in Patients with Psoriasis Treated with Calcipotriol Cream Terapia De Refuerzo En Pacientes Con Psoriasis Tratada Con Calcipotriol [Terapia de refuerzo en pacientes con psoriasis tratada con calcipotriol]. Actas Dermo Sifiliograficas 1998;89(11):626‐30. [EMBASE: 1998399783]
Finlay 1994
-
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) ‐ a simple practical measure for routine clinical use. Clinical & Experimental Dermatology 1994;19(3):210‐6. [MEDLINE: ] - PubMed
Finlay 1995a
-
- Finlay AY, Coles EC. The effect of severe psoriasis on the quality of life of 369 patients. British Journal of Dermatology 1995;132(2):236‐44. [MEDLINE: ] - PubMed
Finlay 2001
-
- Finlay AY. Psoriasis from the patient's point of view. Archives of Dermatology 2001;137(3):352‐3. [MEDLINE: ] - PubMed
Finlay 2005
-
- Finlay AY. Current severe psoriasis and the rule of tens. British Journal of Dermatology 2005;152(5):861‐7. [MEDLINE: ] - PubMed
Floden 1975
-
- Floden CH, Woodbridge P, Samman P, Kurwa AR. Comparison of the Response of Psoriasis, over a 6‐Month Period, to Clobetasol Propionate and Fluocinolone Acetonide Ointments. Current Medical Research & Opinion 1975;3(6):375‐81. [MEDLINE: ] - PubMed
Fluhr 2008
-
- Fluhr JW, Cavallotti C, Berardesca E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clinics in Dermatology 2008; Vol. 26, issue 4:380‐6. [MEDLINE: ] - PubMed
Fortune 2003
-
- Fortune DG, Richards HL, Kirby B, McElhone K, Markham T, Rogers S, et al. Psychological distress impairs clearance of psoriasis in patients treated with photochemotherapy. Archives of Dermatology 2003;139(6):752‐6. [MEDLINE: ] - PubMed
Fouere 2005
-
- Fouere S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 3):2‐6. [MEDLINE: ] - PubMed
Franssen 1999
-
- Franssen ME, Wilt GJ, Jong PC, Bos RP, Arnold WP. A retrospective study of the teratogenicity of dermatological coal tar products. Acta Dermato‐Venereologica 1999;79(5):390‐1. [MEDLINE: ] - PubMed
Fredriksson 1980
-
- Fredriksson T, Lassus A, Bleeker J. Treatment of psoriasis and atopic dermatitis with halcinonide cream applied once and three times daily. British Journal of Dermatology 1980;102(5):575‐7. [MEDLINE: ] - PubMed
Genetic Analysis of Psoriasis Consortium 2010
-
- Genetic Analysis of Psoriasis Consortium, the Wellcome Trust Case Control Consortium, Strange A, Capon F, Spencer CC, Knight J, Weale ME, et al. A genome‐wide association study identifies new psoriasis susceptibility loci and an interaction between HLA‐C and ERAP1. Nature Genetics 2010; Vol. 42, issue 11:985‐90. [PUBMED: 20953190] - PMC - PubMed
Gerritsen 2001
-
- Gerritsen MJ, Kerkhof PC, Langner A. Long‐term safety of topical calcitriol 3 microg g(‐1) ointment. British Journal Of Dermatology 2001;144(Suppl 58):17‐9. [MEDLINE: ] - PubMed
Ghoreschi 2007
-
- Ghoreschi K, Weigert C, Rocken M. Immunopathogenesis and role of T cells in psoriasis. Clinics in Dermatology 2007; Vol. 25, issue 6:574‐80. [MEDLINE: ] - PubMed
Goeckerman 1931
-
- Goeckerman WH. Treatment of psoriasis. AMA Archives of Dermatology & Syphilology 1931;24:446‐50.
Gokdemir 2008
-
- Gokdemir G, Ari S, Köslü A. Adherence to treatment in patients with psoriasis vulgaris: Turkish experience. Journal of the European Academy of Dermatology & Venereology 2008;22(3):330‐5. [MEDLINE: ] - PubMed
Griffiths 2003
-
- Griffiths CE. The immunological basis of psoriasis. Journal of the European Academy of Dermatology & Venereology 2003;17(Suppl 2):1‐5. [MEDLINE: ] - PubMed
Griffiths 2004
-
- Griffiths CE. Psoriasis: future research needs and goals for the twenty‐first century. Dermatologic Clinics 2004; Vol. 22, issue 4:493‐9. [MEDLINE: ] - PubMed
Griffiths 2007
-
- Griffiths CE, Christophers E, Barker JN, Chalmers RJ, Chimenti S, Krueger GG, et al. A classification of psoriasis vulgaris according to phenotype. British Journal of Dermatology 2007; Vol. 156, issue 2:258‐62. [MEDLINE: ] - PubMed
Griffiths 2007a
-
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; Vol. 370, issue 9583:263‐71. [MEDLINE: ] - PubMed
Gupta 1998
-
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis 1998;61(6):339‐42. [MEDLINE: ] - PubMed
Gupta 2007
-
- Gupta G, Bhattacharya S. Psoriasis: maximizing topical treatment concordance. British Journal of Hospital Medicine 2007;68(5):263‐6. [MEDLINE: ] - PubMed
Guzzo 1996
-
- Guzzo C, Lazarus G, Goffe BS, Katz HI, Lowe NJ, Pincus SH. Topical Calcipotriene Has No Short‐Term Effect on Calcium and Bone Metabolism of Patients with Psoriasis. Journal of the American Academy of Dermatology 1996;34(3):429‐33. [MEDLINE: ] - PubMed
Harrington 1995
-
- Harrington CI. Cost‐Effectiveness Analysis of Calcipotriol Ointment and 'Short‐Contact' Dithranol in Treating Mild‐to‐Moderate Psoriasis. British Journal Of Medical Economics 1995;8(1):27‐32. [EMBASE: 1995157081]
Hellgren 1967
-
- Hellgren L. Psoriasis. The prevalence in sex, age and occupational groups in total populations in Sweden: morphology, inheritance and association with other skin and rheumatic diseases. Stockholm: Almqvist & Wiksell, 1967.
Heng 1990
-
- Heng MC, Heng Hl, Allen SG. Basement Membrane Changes in Psoriatic Patients on Long‐Term Topical Corticosteroid Therapy. Clinical & Experimental Dermatology 1990;15(2):83‐90. [MEDLINE: ] - PubMed
Hengge 2006
-
- Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. Journal of the American Academy of Dermatology 2006;54(1):1‐15; quiz 16‐8. [MEDLINE: ] - PubMed
Henseler 1992
-
- Henseler T, Koch F, Westphal E, Nair RP, Vorhees JJ, Elder JT, et al. Presence of HLA‐DR7 in type‐I‐psoriasis. Clinical Research 1992;40:A457.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holick 1996
-
- Holick MF, Chen ML, Kong XF, Sanan DK. Clinical uses for calciotropic hormones 1, 25‐dihydroxyvitamin D3 and parathyroid hormone‐related peptide in dermatology: a new perspective. Journal of Investigative Dermatology. Symposium Proceedings 1996;1(1):1‐9. [MEDLINE: ] - PubMed
Hong 2010
-
- Hong E, Smith SD, Fischer G. Evaluation of the atrophogenic potential of topical corticosteroid in dermatology paediatric patients [Abstract]. 43rd Annual Scientific Meeting of the Australasian College of Dermatologists Darwin, NT Australia, 16‐19 May 2010. Australasian Journal of Dermatology 2010; Vol. 51:A17. [EMBASE: 70162819; 0004‐8380]
Hong 2011
-
- Hong E, Smith S, Fischer G. Evaluation of the atrophogenic potential of topical corticosteroids in pediatric dermatology patients. Pediatric Dermatology 2011; Vol. 28, issue 4:393‐6. [MEDLINE: ; 1525‐1470] - PubMed
Ingram 1953
Jacobi 2008
-
- Jacobi A, Braeutigam M, Mahler V, Schultz E, Hertl M. Pimecrolimus 1% cream in the treatment of facial psoriasis: a 16‐week open‐label study. Dermatology 2008; Vol. 216, issue 2:133‐6. [MEDLINE: ; 1421‐9832] - PubMed
Jales 2012
Jenner 2002
-
- Jenner N, Campbell J, Plunkett A, Marks R. Cost of psoriasis: a study on the morbidity and financial effects of having psoriasis in Australia. Australasian Journal of Dermatology 2002;43(4):255‐61. [MEDLINE: ] - PubMed
Juni 2001
Kao 2003
-
- Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short‐term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. Journal of Investigative Dermatology 2003; Vol. 120, issue 3:456‐64. [MEDLINE: ] - PubMed
Katz 1987b
-
- Katz HI, Hien NT, Prawer SE, Mastbaum LI, Mooney JJ, Samson CR. Superpotent Topical Steroid Treatment of Psoriasis Vulgaris‐‐Clinical Efficacy and Adrenal Function. Journal of the American Academy of Dermatology 1987;16(4):804‐11. [MEDLINE: ] - PubMed
Katz 1989
-
- Katz HI, Prawer SE, Mooney JJ, Samson CR. Preatrophy: Covert Sign of Thinned Skin. Journal of the American Academy of Dermatology 1989;20(5 Pt 1):731‐5. [MEDLINE: ] - PubMed
Kimball 2008
-
- Kimball AB, Gold MH, Zib B, Davis MW, Clobetasol Propionate Emulsion Formulation Foam Phase IIICSG. Clobetasol propionate emulsion formulation foam 0.05%: review of phase II open‐label and phase III randomized controlled trials in steroid‐responsive dermatoses in adults and adolescents. Journal of the American Academy of Dermatology 2008; Vol. 59, issue 3:448‐54. [MEDLINE: ; 1097‐6787] - PubMed
Kragballe 1988
-
- Kragballe K, Beck HI, Søgaard H. Improvement of psoriasis by a topical vitamin D3 analogue (Mc 903) in a double‐blind study. British Journal of Dermatology 1988;119(2):223‐30. [MEDLINE: ] - PubMed
Kragballe 1991b
-
- Kragballe K, Fogh K, Sogaard H. Long‐Term Efficacy and Tolerability of Topical Calcipotriol in Psoriasis. Results of an Open Study. Acta Dermato‐Venereologica 1991;71(6):475‐8. [MEDLINE: ] - PubMed
Krueger 1984
-
- Krueger GG, Bergstresser PR, Lowe NJ, Voorhees JJ, Weinstein GD. Psoriasis. Journal of the American Academy of Dermatology 1984;11(5 Pt 2):937‐47. [MEDLINE: ] - PubMed
Krueger 2000
-
- Krueger GG, Feldman SR, Camisa C, Duvic M, Elder JT, Gottlieb AB, et al. Two considerations for patients with psoriasis and their clinicians: what defines mild, moderate, and severe psoriasis? What constitutes a clinically significant improvement when treating psoriasis?. Journal of the American Academy of Dermatology 2000;43(2 Pt 1):281‐5. [MEDLINE: ] - PubMed
Lambert 1996
-
- Lambert JRMG. Economic Analyses of the Treatment of Psoriasis. European Journal of Dermatology 1996;6(8):543‐7. [EMBASE: 1997003685]
Lambert 1999
-
- Lambert JRMG. Cost‐Effectiveness of Treatments in Psoriasis. Journal Of Dermatological Treatment 1999;10(Suppl 1):S9‐S13. [EMBASE: 1999115355]
Lambert 2002
-
- Lambert J, Trompke C. Tacalcitol ointment for long‐term control of chronic plaque psoriasis in dermatological practice. Dermatology 2002;204(4):321‐4. [MEDLINE: ] - PubMed
Langner 1996
-
- Langner A, Ashton P, Kerkhof PC, Verjans H. A long‐term multicentre assessment of the safety and tolerability of calcitriol ointment in the treatment of chronic plaque psoriasis. British Journal of Dermatology 1996;135(3):385‐9. [MEDLINE: ] - PubMed
Lebwohl 1996
-
- Lebwohl M, Siskin SB, Epinette W, Breneman D, Funicella T, Kalb R, et al. A Multicenter Trial of Calcipotriene Ointment and Halobetasol Ointment Compared with Either Agent Alone for the Treatment of Psoriasis. Journal of the American Academy of Dermatology 1996;35(2 Pt 1):268‐9. [MEDLINE: ] - PubMed
Lee 1990
-
- Lee FI, Bellary SV, Francis C. Increased occurrence of psoriasis in patients with Crohn's disease and their relatives [comment]. American Journal of Gastroenterology 1990;85(8):962‐3. [MEDLINE: ] - PubMed
Lee 1998
-
- Lee JY, Maibach HI. Corticosteroid skin atrophogenicity: assessment methods. Skin Research & Technology 1998;4(4):161‐6. [EMBASE: 1998377637] - PubMed
Lee 2006
-
- Lee IA, Maibach HI. Pharmionics in dermatology: a review of topical medication adherence. American Journal of Clinical Dermatology 2006;7(4):231‐6. [MEDLINE: ] - PubMed
Leu 1985
-
- Leu RE. Economic Evaluation of New Drug Therapies in Terms of Improved Life Quality. Social Science & Medicine 1985;21(10):1153‐61. [MEDLINE: ] - PubMed
Lomholt 1963
-
- Lomholt G. Psoriasis, prevalence, spontaneous course and genetics. Published Ph.D Thesis. Copenhagen: G.E.C. Gad, 1963:31‐3.
Lundberg 1999
-
- Lundberg L, Johannesson M, Silverdahl M, Hermansson C, Lindberg M. Quality of life, health‐state utilities and willingness to pay in patients with psoriasis and atopic eczema. British Journal of Dermatology 1999;141(6):1067‐75. [MEDLINE: ] - PubMed
Marchetti 1998
-
- Marchetti A, Pensee K, An P. A Pharmacoeconomic Analysis of Topical Therapies for Patients with Mild‐to‐Moderate Stable Plaque Psoriasis: A US Study. Clinical Therapeutics 1998;20(4):851‐69. [MEDLINE: ] - PubMed
Matthews 1996
-
- Matthews D, Fry L, Powles A, Weber J, McCarthy M, Fisher E, et al. Evidence that a locus for familial psoriasis maps to chromosome 4q. Nature Genetics 1996;14(2):231‐3. [MEDLINE: ] - PubMed
McKenna 2003
-
- McKenna SP, Cook SA, Whalley D, Doward LC, Richards HL, Griffiths CE, et al. Development of the PSORIQoL, a psoriasis‐specific measure of quality of life designed for use in clinical practice and trials. British Journal of Dermatology 2003;149(2):323‐31. [MEDLINE: ] - PubMed
Mee 1998
-
- Mee JB, Cork MJ. Vitamin D receptor polymorphism and calcipotriol response in patients with psoriasis. Journal of Investigative Dermatology 1998;110(3):301‐2. [MEDLINE: ] - PubMed
Menter 2007
-
- Menter A. Topical monotherapy with clobetasol propionate spray 0.05% in the COBRA trial. Cutis 2007;80(5 Suppl):12‐9. [MEDLINE: ; 0011‐4162] - PubMed
Miyachi 2002
-
- Miyachi Y, Ohkawara A, Ohkido M, Harada S, Tamaki K, Nakagawa H, et al. Long‐term safety and efficacy of high‐concentration (20 microg/g) tacalcitol ointment in psoriasis vulgaris. European Journal of Dermatology 2002;12(5):463‐8. [MEDLINE: ] - PubMed
Mortensen 1993
-
- Mortensen L, Kragballe K, Wegmann E, Schifter S, Risteli J, Charles P. Treatment of psoriasis vulgaris with topical calcipotriol has no short‐term effect on calcium or bone metabolism. A randomized, double‐blind, placebo‐controlled study. Acta Dermato‐Venereologica 1993;73(4):300‐4. [MEDLINE: ] - PubMed
Nair 1997
-
- Nair RP, Henseler T, Jenisch S, Stuart P, Bichakjian CK, Lenk W, et al. Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) by genome‐wide scan. Human Molecular Genetics 1997;6(8):1349‐56. [MEDLINE: ] - PubMed
NHSCRD 2001
-
- NHS Centre for Reviews & Dissemination. Undertaking Systematic Reviews of Research on Effectiveness: CRD guidelines for those Carrying Out or Commissioning Reviews. 2nd Edition. Vol. CRD Report Number 4 (2nd edition), York: CRD, 2001.
Oh 1997
-
- Oh PI, Gupta AK, Einarson TR, Maerov P, Shear NH. Calcipotriol in the Treatment of Psoriasis of Limited Severity: Pharmacoeconomic Evaluation. Journal of Cutaneous Medicine & Surgery 1997;2(1):7‐15. [EMBASE: 1998192329]
Ortonne 2000
-
- Ortonne JP. Psoriasis: evaluation of quality of life. Annales de Dermatologie et de Venereologie 2000;127(Suppl 2):2s19‐22. [MEDLINE: ] - PubMed
Osborne 2002
-
- Osborne JE, Hutchinson PE. The importance of accurate dosage of topical agents: a method of estimating involved area and application to calcipotriol treatment failures. Journal of the European Academy of Dermatology & Venereology 2002;16(4):367‐73. [MEDLINE: ] - PubMed
Owen 1993
-
- Owen OG. Healthy returns come from costly drug ‐ prescribing an expensive drug may be the most cost‐effective way to treat patients. Healthcare Management 1993;January:52‐3.
Owen 2000
Park 2002
-
- Park YK, Lee JH, Chung WG. Allergic contact dermatitis from calcipotriol. Acta Dermato‐Venereologica 2002;82(1):71‐2. [MEDLINE: ] - PubMed
Parodi 1991
-
- Parodi A, Guarrera M, Volonte MV. Medicated Tape Versus Cream Formulation ‐ a Major Saving of Fluocinolone Acetonide. Journal Of Dermatological Treatment 1991;1(6):305‐6. [EMBASE: 1991180520]
Poyner 1993
-
- Poyner T, Hughes IW, Dass BK, Adnitt PI. Long‐Term Treatment of Chronic Plaque Psoriasis with Calcipotriol. Journal Of Dermatological Treatment 1993;4(4):173‐7. [EMBASE: 1994040748]
Poyner 1999
-
- Poyner TF, Wall A, Adnitt PI, Menday AP. Economic Impact of Psoriasis Treatment on the Patient and on the National Health Service. Journal Of Dermatological Treatment 1999;10(1):25‐9. [EMBASE: 1999115428]
Poyner 2000
-
- Poyner TF, Menday AP, Williams ZV. Patient attitudes to topical antipsoriatic treatment with calcipotriol and dithranol. Journal of the European Academy of Dermatology & Venereology 2000;14(3):153‐8. [MEDLINE: ] - PubMed
Ramsay 1994
-
- Ramsay CA, Berth‐Jones J, Brundin G, Cunliffe WJ, Dubertret L, Kerkhof PC, et al. Long‐Term Use of Topical Calcipotriol in Chronic Plaque Psoriasis. Dermatology 1994;189(3):260‐4. [PUBMED: 7949479] - PubMed
Richards 1999
-
- Richards HL, Fortune DG, O'Sullivan TM, Main CJ, Griffiths CE. Patients with psoriasis and their compliance with medication. Journal of the American Academy of Dermatology 1999;41(4):581‐3. [MEDLINE: ] - PubMed
Richards 2003
-
- Richards HL, Fortune DG, Main CJ, Griffiths CE. Stigmatization and psoriasis. British Journal of Dermatology 2003;149(1):209‐11. [MEDLINE: ] - PubMed
Richards 2006
-
- Richards HL, Fortune DG, Griffiths CE. Adherence to treatment in patients with psoriasis. Journal of the European Academy of Dermatology & Venereology 2006;20(4):370‐9. [MEDLINE: ] - PubMed
Roberts 2011
-
- Roberts C, Angus JE, Williams HC, Villanueva E, Saeterdal I, Jobling R. Ustekinumab for plaque psoriasis. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008947] - DOI
Roelofzen 2010
-
- Roelofzen JH, Aben KK, Oldenhof UT, Coenraads PJ, Alkemade HA, Kerkhof PC, et al. No increased risk of cancer after coal tar treatment in patients with psoriasis or eczema. Journal of Investigative Dermatology 2010; Vol. 130, issue 4:953‐61. [MEDLINE: ; 1523‐1747] - PubMed
Russell 1972
-
- Russell TJ, Schultes LM, Kuban DJ. Histocompatibility (HL‐A) antigens associated with psoriasis. New England Journal of Medicine 1972;287(15):738‐40. [MEDLINE: ] - PubMed
Salvarani 1995
-
- Salvarani C, Lo Scocco G, Macchioni P, Cremonesi T, Rossi F, Mantovani W, et al. Prevalence of psoriatic arthritis in Italian psoriatic patients. Journal of Rheumatology 1995;22(8):1499‐503. [MEDLINE: ] - PubMed
Schiffner 2003
-
- Schiffner R, Schiffner‐Rohe J, Gerstenhauer M, Hofstadter F, Landthaler M, Stolz W. Willingness to pay and time trade‐off: sensitive to changes of quality of life in psoriasis patients?. British Journal of Dermatology 2003;148(6):1153‐60. [MEDLINE: ] - PubMed
Schwicker 1992
-
- Schwicker D, Dinkel R, Antunes HC. A Cost‐Comparison Study: Ulobetasol Versus Clobetasol in Severe Localized Psoriasis. Journal Of Dermatological Treatment 1992;2(4):127‐31. [EMBASE: 1992107343] - PubMed
Senter 1983
-
- Senter TP. Topical fluocinonide and tachyphylaxis letter. Archives of Dermatology 1983;119(5):363‐4. [MEDLINE: ] - PubMed
Simons 1949
-
- Simons RD. Additional studies on psoriasis in the tropics and in starvation camps. Journal of Investigative Dermatology 1949;12(5):285‐94. [MEDLINE: ] - PubMed
Singh 2000
-
- Singh S, Reddy DC, Pandey SS. Topical therapy for psoriasis with the use of augmented betamethasone and calcipotriene on alternate weeks. Journal of the American Academy of Dermatology 2000;43(1 Pt 1):61‐5. [MEDLINE: ] - PubMed
Sorensen 2002
-
- Sorensen M, Norregaard J. Pharmacoeconomic evaluation of a new two compound ointment (Daivobet (R)) and calcipotriol (Daivonex (R)) in the treatment of psoriasis vulgaris in Sweden. Value Health 2002;5(6):554‐5.
Stern 1988
-
- Stern RS. The Benefits, Costs and Risks of Topical Tar Preparations in the Treatment of Psoriasis: Considerations of Cost Effectiveness. Annals Of The Academy Of Medicine, Singapore 1988;17(4):473‐6. [MEDLINE: ] - PubMed
Stern 1995
-
- Stern RS. Epidemiology of psoriasis. Dermatologic Clinics 1995;13(4):717‐22. [MEDLINE: ] - PubMed
Stern 1997
-
- Stern RS. Psoriasis. Lancet 1997;350(9074):349‐53. [MEDLINE: ] - PubMed
Stevanovic 1977
-
- Stevanovic DV, Wilson L, Sparkes CG. A separation of clinical from epidermal thinning effect in the topical glucocorticoid clobetasone butyrate. British Journal of Dermatology 1977;96(1):67‐70. [MEDLINE: ] - PubMed
Svejgaard 1974
-
- Svejgaard A, Nielsen LS, Svejgaard E, Nielsen FK, Hjortshoj A, Zachariae H. HL‐A in psoriasis vulgaris and in pustular psoriasis ‐ population and family studies. British Journal of Dermatology 1974;91(2):145‐53. [MEDLINE: ] - PubMed
Szeimies 2004
-
- Szeimies RM. Psoriasis: Patient‐oriented treatment improves compliance [Psoriasis: Patientenorientierte behandlung verbessert compliance]. Haut 2004;15(5):221‐3. [EMBASE: 2004422749]
Tagami 1997
-
- Tagami H. Triggering factors. Clinics in Dermatology 1997;15(5):677‐85. [MEDLINE: ] - PubMed
Taylor 2006
-
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis & Rheumatism 2006; Vol. 54, issue 8:2665‐73. [MEDLINE: ] - PubMed
Tazi‐Ahnini 1999a
-
- Tazi Ahnini R, Camp NJ, Cork MJ, Mee JB, Keohane SG, Duff GW, et al. Novel genetic association between the corneodesmosin (MHC S) gene and susceptibility to psoriasis. Human Molecular Genetics 1999;8(6):1135‐40. [MEDLINE: ] - PubMed
Tazi‐Ahnini 1999b
-
- Tazi‐Ahnini R, Giovine FS, Cox A, Keohane SG, Cork MJ. Corneodesmosin (MHC S) gene in guttate psoriasis. Lancet 1999;354(9178):597. [MEDLINE: ] - PubMed
Tomfohrde 1994
-
- Tomfohrde J, Silverman A, Barnes R, Fernandez‐Vina MA, Young M, Lory D, et al. Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 1994;264(5162):1141‐5. [MEDLINE: ] - PubMed
Traulsen 2003
-
- Traulsen J, Hughes‐Formella BJ. The atrophogenic potential and dermal tolerance of calcipotriol/betamethasone dipropionate ointment compared with betamethasone dipropionate ointment. Dermatology 2003;207(2):166‐72. [MEDLINE: ] - PubMed
Trembath 1997
-
- Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RD, Frodsham A, et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome‐wide search in psoriasis. Human Molecular Genetics 1997;6(5):813‐20. [MEDLINE: ] - PubMed
Tsoi 2012
Uhoda 2003
-
- Uhoda I, Quatresooz P, Hermanns‐Le T, Pierard‐Franchimont C, Arrese JE, Pierard GE. Histometric assessment of psoriatic plaques treated by vitamin D3 derivatives. Dermatology 2003;206(4):366‐9. [MEDLINE: ] - PubMed
Unna 1916
-
- Unna PG. Cignolin als Heilmittel der Psoriasis. Dermatologische Wochenschrift 1916;62:116‐37.
van de Kerkhof 1996c
-
- Kerkhof PCM, Harten J, Verjans H. A Long‐Term Assessment of the Safety and Tolerability of Calcitriol Ointment in the Treatment of Chronic Plaque Psoriasis. Journal Of Dermatological Treatment 1996;7(Suppl 1):S11‐14. [EMBASE: 1996266413]
van de Kerkhof 1997a
-
- Kerkhof PC. Combinations and comparisons. Clinics in Dermatology 1997;15(5):831‐4. [MEDLINE: ] - PubMed
van de Kerkhof 1997b
-
- Kerkhof P, Vleuten C, Gerritsen M, Glade C, Luger T, Werfel T, et al. Long‐Term Efficacy and Safety of Once Daily Treatment of Chronic Plaque Psoriasis with Tacalcitol Ointment. European Journal of Dermatology 1997;7(6):421‐5. [EMBASE: 1997293421]
van de Kerkhof 1998
-
- Kerkhof PC. An update on vitamin D3 analogues in the treatment of psoriasis. Skin Pharmacology & Applied Skin Physiology 1998;11(1):2‐10. [MEDLINE: ] - PubMed
van de Kerkhof 1998c
-
- Kerkhof PCM, Steegers Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology 1998;197(1):31‐6. [MEDLINE: ] - PubMed
van de Kerkhof 2000
-
- Kerkhof PC, Hoop D, Korte J, Cobelens SA, Kuipers MV. Patient compliance and disease management in the treatment of psoriasis in the Netherlands. Dermatology 2000;200(4):292‐8. [MEDLINE: ] - PubMed
van de Kerkhof 2001
-
- Kerkhof PC, Franssen M, Brassine M, Kuipers M. Calcipotriol cream in the morning and ointment in the evening: a novel regimen to improve compliance. Journal of Dermatological Treatment 2001;12(2):75‐9. [MEDLINE: ] - PubMed
van de Kerkhof 2002c
-
- Kerkhof PC, Berth‐Jones J, Griffiths CE, Harrison PV, Honigsmann H, Marks R, et al. Long‐term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. British Journal of Dermatology 2002;146(3):414‐22. [MEDLINE: ] - PubMed
van de Kerkhof 2004
-
- Kerkhof PC. The impact of a two‐compound product containing calcipotriol and betamethasone dipropionate (Daivobet/ Dovobet) on the quality of life in patients with psoriasis vulgaris: a randomized controlled trial. British Journal of Dermatology 2004;151(3):663‐8. [MEDLINE: ] - PubMed
Van de Kerkhof 2008
-
- Kerkhof PC, Barker J, Griffiths CE, Kragballe K, Mason J, Menter A, et al. Psoriasis: Consensus on topical therapies. Journal of the European Academy of Dermatology & Venereology 2008; Vol. 22, issue 7:859‐70. [MEDLINE: ] - PubMed
Vazquez‐Lopez 2004
-
- Vazquez‐Lopez F, Marghoob AA. Dermoscopic assessment of long‐term topical therapies with potent steroids in chronic psoriasis. Journal of the American Academy of Dermatology 2004;51(5):811‐3. [MEDLINE: ] - PubMed
Velema 2009
Veraldi 2006
-
- Veraldi S, Caputo R, Pacifico A, Peris K, Soda R, Chimenti S. Short contact therapy with tazarotene in psoriasis vulgaris. Dermatology 2006; Vol. 212, issue 3:235‐7. [MEDLINE: ; 1018‐8665] - PubMed
Vissers 2004
-
- Vissers WH, Berends M, Muys L, Erp PE, Jong EM, Kerkhof PC. The effect of the combination of calcipotriol and betamethasone dipropionate versus both monotherapies on epidermal proliferation, keratinization and T‐cell subsets in chronic plaque psoriasis. Experimental Dermatology 2004;13(2):106‐12. [MEDLINE: ] - PubMed
Watts 1998
-
- Watts J. Helping people to remain in control of their psoriasis. Community Nurse 1998;4(7):19‐21. [MEDLINE: ] - PubMed
Willan 1808
-
- Willan R. On cutaneous diseases. London: Johnson, 1808.
Wishart 1994
-
- Wishart JM. Calcitriol (1 alpha,25‐dihydroxyvitamin D3) ointment in psoriasis, a safety tolerance and efficacy multicentre study. Dermatology 1994; Vol. 188, issue 2:135‐9. [MEDLINE: ; 1018‐8665] - PubMed
Yip 1984
-
- Yip SY. The prevalence of psoriasis in the Mongoloid race. Journal of the American Academy of Dermatology 1984;10(6):965‐8. [MEDLINE: ] - PubMed
Zachariae 2002
-
- Zachariae R, Zachariae H, Blomqvist K, Davidsson S, Molin L, Mork C, et al. Quality of life in 6497 Nordic patients with psoriasis. British Journal of Dermatology 2002;146(6):1006‐16. [MEDLINE: ] - PubMed
Zaghloul 2004
-
- Zaghloul SS, Goodfield MJ. Objective assessment of compliance with psoriasis treatment. Archives of Dermatology 2004;140(4):408‐14. [MEDLINE: ] - PubMed
Zanolli 1992
-
- Zanolli MD, Wikle JS. Joint complaints in psoriasis patients. International Journal of Dermatology 1992;31(7):488‐91. [MEDLINE: ] - PubMed
Zug 1995
-
- Zug KA, Littenberg B, Baughman RD, Kneeland T, Nease RF, Sumner W, et al. Assessing the Preferences of Patients with Psoriasis: A Quantitative, Utility Approach. Archives of Dermatology 1995;131(5):561‐8. [MEDLINE: ] - PubMed
References to other published versions of this review
Mason 2002a
-
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. British Journal Of Dermatology 2002;146(3):351‐64. [MEDLINE: ] - PubMed
Mason 2002b
-
- Mason J, Mason A, Cork M. Topical preparations for the treatment of psoriasis in primary care: a systematic review. University of York (OP41). York: University of York, 2002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

