Morpho-functional architecture of the Golgi complex of neuroendocrine cells
- PMID: 23543640
- PMCID: PMC3610015
- DOI: 10.3389/fendo.2013.00041
Morpho-functional architecture of the Golgi complex of neuroendocrine cells
Abstract
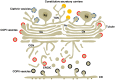
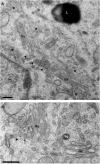
In neuroendocrine cells, prohormones move from the endoplasmic reticulum to the Golgi complex (GC), where they are sorted and packed into secretory granules. The GC is considered the central station of the secretory pathway of proteins and lipids en route to their final destination. In most mammalian cells, it is formed by several stacks of cisternae connected by tubules, forming a continuous ribbon. This organelle shows an extraordinary structural and functional complexity, which is exacerbated by the fact that its architecture is cell type specific and also tuned by the functional status of the cell. It is, indeed, one the most beautiful cellular organelles and, for that reason, perhaps the most extensively photographed by electron microscopists. In recent decades, an exhaustive dissection of the molecular machinery involved in membrane traffic and other Golgi functions has been carried out. Concomitantly, detailed morphological studies have been performed, including 3D analysis by electron tomography, and the precise location of key proteins has been identified by immunoelectron microscopy. Despite all this effort, some basic aspects of Golgi functioning remain unsolved. For instance, the mode of intra-Golgi transport is not known, and two opposing theories (vesicular transport and cisternal maturation models) have polarized the field for many years. Neither of these theories explains all the experimental data so that new theories and combinations thereof have recently been proposed. Moreover, the specific role of the small vesicles and tubules which surround the stacks needs to be clarified. In this review, we summarize our current knowledge of the Golgi architecture in relation with its function and the mechanisms of intra-Golgi transport. Within the same framework, the characteristics of the GC of neuroendocrine cells are analyzed.
Keywords: golgi complex; intra-golgi transport; morphology; neuroendocrine cells; transport vesicles; tubules.
Figures

Similar articles
-
Role of microtubules in the organization of the Golgi complex.Exp Cell Res. 1999 Feb 1;246(2):263-79. doi: 10.1006/excr.1998.4326. Exp Cell Res. 1999. PMID: 9925741 Review.
-
Prohormone transport through the secretory pathway of neuroendocrine cells.Biochem Cell Biol. 2000;78(3):289-98. Biochem Cell Biol. 2000. PMID: 10949080 Review.
-
Structural Organization and Function of the Golgi Ribbon During Cell Division.Front Cell Dev Biol. 2022 Jun 24;10:925228. doi: 10.3389/fcell.2022.925228. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35813197 Free PMC article.
-
Architecture of the mammalian Golgi.Cold Spring Harb Perspect Biol. 2011 Jul 1;3(7):a005181. doi: 10.1101/cshperspect.a005181. Cold Spring Harb Perspect Biol. 2011. PMID: 21502307 Free PMC article. Review.
-
Connections between the various elements of the cis- and mid-compartments of the Golgi apparatus of early rat spermatids.Anat Rec. 1994 Dec;240(4):469-80. doi: 10.1002/ar.1092400405. Anat Rec. 1994. PMID: 7879899
Cited by
-
The Golgin Protein Giantin Regulates Interconnections Between Golgi Stacks.Front Cell Dev Biol. 2019 Aug 27;7:160. doi: 10.3389/fcell.2019.00160. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31544102 Free PMC article.
-
The regulated secretory pathway in neuroendocrine cells.Front Endocrinol (Lausanne). 2014 Apr 8;5:48. doi: 10.3389/fendo.2014.00048. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 24782828 Free PMC article. No abstract available.
-
Changes in the content of pollen total lipid and TAG in Arabidopsis thaliana DGAT1 mutant as11.AoB Plants. 2023 Mar 22;15(2):plad012. doi: 10.1093/aobpla/plad012. eCollection 2023 Feb. AoB Plants. 2023. PMID: 37064996 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

