Loss of calcium/calmodulin-dependent protein kinase II activity in cortical astrocytes decreases glutamate uptake and induces neurotoxic release of ATP
- PMID: 23543737
- PMCID: PMC3656312
- DOI: 10.1074/jbc.M113.466235
Loss of calcium/calmodulin-dependent protein kinase II activity in cortical astrocytes decreases glutamate uptake and induces neurotoxic release of ATP
Abstract
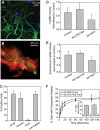
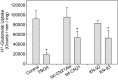
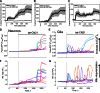
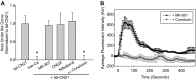
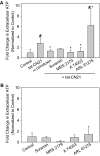
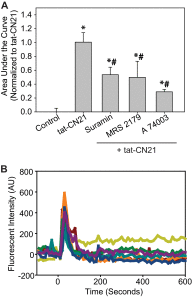
The extent of calcium/calmodulin-dependent protein kinase II (CaMKII) inactivation in the brain after ischemia correlates with the extent of damage. We have previously shown that a loss of CaMKII activity in neurons is detrimental to neuronal viability by inducing excitotoxic glutamate release. In the current study we extend these findings to show that the ability of astrocytes to buffer extracellular glutamate is reduced when CaMKII is inhibited. Furthermore, CaMKII inhibition in astrocytes is associated with the rapid onset of intracellular calcium oscillations. Surprisingly, this rapid calcium influx is blocked by the N-type calcium channel antagonist, ω-conotoxin. Although the function of N-type calcium channels within astrocytes is controversial, these voltage-gated calcium channels have been linked to calcium-dependent vesicular gliotransmitter release. When extracellular glutamate and ATP levels are measured after CaMKII inhibition within our enriched astrocyte cultures, no alterations in glutamate levels are observed, whereas ATP levels in the extracellular environment significantly increase. Extracellular ATP accumulation associated with CaMKII inhibition contributes both to calcium oscillations within astrocytes and ultimately cortical neuron toxicity. Thus, a loss of CaMKII signaling within astrocytes dysregulates glutamate uptake and supports ATP release, two processes that would compromise neuronal survival after ischemic/excitotoxic insults.
Keywords: CaMKII; Calcium Signaling; Glia; Glutamate; Neurodegeneration; Neuronal-Glial Communication.
Figures



References
-
- Sakagami H., Watanabe M., Kondo H. (1992) Gene expression of Ca2+/calmodulin-dependent protein kinase of the cerebellar granule cell type or type IV in the mature and developing rat brain. Brain. Res. Mol. Brain. Res. 16, 20–28 - PubMed
-
- Bayer K. U., Löhler J., Schulman H., Harbers K. (1999) Developmental expression of the CaM kinase II isoforms. Ubiquitous γ- and δ-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain. Res. Mol. Brain. Res. 70, 147–154 - PubMed
-
- McGuinness T. L., Lai Y., Greengard P. (1985) Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J. Biol. Chem. 260, 1696–1704 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

