Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons
- PMID: 23544095
- PMCID: PMC3609769
- DOI: 10.1371/journal.pone.0059759
Light-emitting channelrhodopsins for combined optogenetic and chemical-genetic control of neurons
Abstract
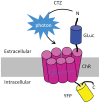
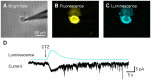
Manipulation of neuronal activity through genetically targeted actuator molecules is a powerful approach for studying information flow in the brain. In these approaches the genetically targeted component, a receptor or a channel, is activated either by a small molecule (chemical genetics) or by light from a physical source (optogenetics). We developed a hybrid technology that allows control of the same neurons by both optogenetic and chemical genetic means. The approach is based on engineered chimeric fusions of a light-generating protein (luciferase) to a light-activated ion channel (channelrhodopsin). Ionic currents then can be activated by bioluminescence upon activation of luciferase by its substrate, coelenterazine (CTZ), as well as by external light. In cell lines, expression of the fusion of Gaussia luciferase to Channelrhodopsin-2 yielded photocurrents in response to CTZ. Larger photocurrents were produced by fusing the luciferase to Volvox Channelrhodopsin-1. This version allowed chemical modulation of neuronal activity when expressed in cultured neurons: CTZ treatment shifted neuronal responses to injected currents and sensitized neurons to fire action potentials in response to subthreshold synaptic inputs. These luminescent channelrhodopsins--or luminopsins--preserve the advantages of light-activated ion channels, while extending their capabilities. Our proof-of-principle results suggest that this novel class of tools can be improved and extended in numerous ways.
Conflict of interest statement
Figures




References
-
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011) Optogenetics in neural systems. Neuron 71: 9–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

