Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials
- PMID: 23544105
- PMCID: PMC3609729
- DOI: 10.1371/journal.pone.0059872
Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trials
Erratum in
- PLoS One. 2013;8(6). doi: 10.1371/annotation/8da4be4b-2de1-4c51-9c40-0f49dc212579
Abstract
Background: Interleukin (IL)-5 is believed to be a key cytokine in eosinophil inflammatory infiltration in asthma. Previous clinical trials have evaluated the efficacy and safety of mepolizumab, a monoclonal antibody against IL-5, in patients with asthma. However, most of these studies were small, the conclusions were inconsistent, and the precise effects are therefore debatable.
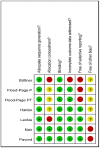
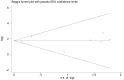
Methods: A meta-analysis of randomized placebo-controlled trials was conducted to evaluate the effect of intravenous infusion of mepolizumab on clinical outcomes in patients with asthma. Trials were searched in PubMed, Embase, Web of Science, Cochrane CENTRAL, Scopus, reviews, and reference lists of relevant articles. The outcome variables analyzed included eosinophil counts in blood and sputum, airways outcome measures, exacerbations, asthma control, and quality of life scores.
Results: Seven studies met final inclusion criteria (total n = 1131). From the pooled analyses, mepolizumab significantly reduced eosinophils in blood (MD -0.29×10(9)/L, 95% CI -0.44 to -0.14×10(9)/L, P = 0.0001) and sputum (MD -6.05%, 95% CI -9.34 to -2.77%, P = 0.0003). Mepolizumab was also associated with significantly decreased exacerbation risk than placebo (OR 0.30, 95%CI 0.13 to 0.67, P = 0.004), and with a significant improvement in the scores on the Asthma Quality of Life Questionnaire (AQLQ) (MD 0.26, 95% CI 0.03 to 0.49, P = 0.03) in patients with eosinophilic asthma. There were no statistical differences between the groups with respect to FEV1, PEF, or histamine PC20 (all P>0.05), and a non-significant trend for improvement in scores on the Juniper Asthma Control Questionnaire (JACQ) (MD -0.21, 95% CI -0.43 to 0.01, P = 0.06) in the mepolizumab group was observed.
Conclusions: Mepolizumab reduces the risk of exacerbations and improves quality of life in patients with eosinophilic asthma, but no significant improvement in lung function outcomes was observed. Further research is required to establish the possible role of anti-IL-5 as a therapy for asthma.
Conflict of interest statement
Figures

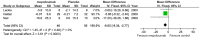
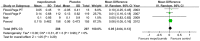
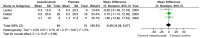
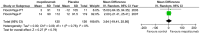
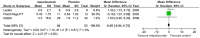
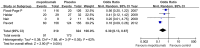
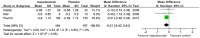
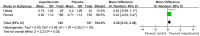
Similar articles
-
Anti-IL-5 therapies for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2020 Dec 8;12(12):CD013432. doi: 10.1002/14651858.CD013432.pub2. Cochrane Database Syst Rev. 2020. PMID: 33295032 Free PMC article.
-
Effect of mepolizumab in severe eosinophilic asthma according to omalizumab eligibility.Respir Med. 2019 Jul-Aug;154:69-75. doi: 10.1016/j.rmed.2019.06.004. Epub 2019 Jun 8. Respir Med. 2019. PMID: 31220806
-
Mepolizumab and exacerbations of refractory eosinophilic asthma.N Engl J Med. 2009 Mar 5;360(10):973-84. doi: 10.1056/NEJMoa0808991. N Engl J Med. 2009. PMID: 19264686 Free PMC article. Clinical Trial.
-
A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma.Am J Respir Crit Care Med. 2007 Dec 1;176(11):1062-71. doi: 10.1164/rccm.200701-085OC. Epub 2007 Sep 13. Am J Respir Crit Care Med. 2007. PMID: 17872493 Clinical Trial.
-
Evidence for the efficacy and safety of anti-interleukin-5 treatment in the management of refractory eosinophilic asthma.Ther Adv Respir Dis. 2015 Aug;9(4):135-45. doi: 10.1177/1753465815581279. Epub 2015 Apr 21. Ther Adv Respir Dis. 2015. PMID: 25900924 Review.
Cited by
-
Very long-term data on patients with severe eosinophilic asthma treated with mepolizumab: a case series.Drugs Context. 2024 Jul 22;13:2024-4-2. doi: 10.7573/dic.2024-4-2. eCollection 2024. Drugs Context. 2024. PMID: 39072303 Free PMC article.
-
GEMA 5.3. Spanish Guideline on the Management of Asthma.Open Respir Arch. 2023 Sep 19;5(4):100277. doi: 10.1016/j.opresp.2023.100277. eCollection 2023 Oct-Dec. Open Respir Arch. 2023. PMID: 37886027 Free PMC article.
-
Stepwise Approach in Asthma Revisited 2023: Expert Panel Opinion of Turkish Guideline of Asthma Diagnosis and Management Group.Thorac Res Pract. 2023 Nov;24(6):309-324. doi: 10.5152/ThoracResPract.2023.23035. Thorac Res Pract. 2023. PMID: 37909830 Free PMC article.
-
Anti-Interleukin 5 (IL-5) and IL-5Ra Biological Drugs: Efficacy, Safety, and Future Perspectives in Severe Eosinophilic Asthma.Front Med (Lausanne). 2017 Aug 31;4:135. doi: 10.3389/fmed.2017.00135. eCollection 2017. Front Med (Lausanne). 2017. PMID: 28913336 Free PMC article. Review.
-
Type 2 inflammation in asthma--present in most, absent in many.Nat Rev Immunol. 2015 Jan;15(1):57-65. doi: 10.1038/nri3786. Nat Rev Immunol. 2015. PMID: 25534623 Free PMC article. Review.
References
-
- Filley WV, Holley KE, Kephart GM, Gleich GJ (1982) Identification by immunofluorescence of eosinophil granule major basic protein in lung tissue of patients with bronchial asthma. Lancet 2: 11–16. - PubMed
-
- Coyle AJ, Ackerman SJ, Irvin CG (1993) Cationic proteins induce airway hyperresponsiveness dependent on charge interactions. Am Rev Respir Dis 147: 896–900. - PubMed
-
- Wardlaw AJ, Brightling CE, Green R, Woltmann G, Bradding P, et al. (2002) New insights into the relationship between airway inflammation and asthma. Clin Sci (Lond) 103: 201–211. - PubMed
-
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, et al. (1990) Eosinophilic inflammation in asthma. N Engl J Med 323: 1033–1039. - PubMed
-
- Sehmi R, Wardlaw AJ, Cromwell O, Kurihara K, Waltmann P, et al. (1992) Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood 79: 2952–2959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

