Increased antigen specific T cell numbers in the absence of altered migration or division rates as a result of mucosal cholera toxin administration
- PMID: 23544110
- PMCID: PMC3609821
- DOI: 10.1371/journal.pone.0059934
Increased antigen specific T cell numbers in the absence of altered migration or division rates as a result of mucosal cholera toxin administration
Abstract
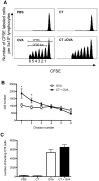
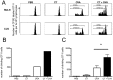
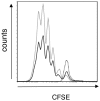
Cholera toxin (CT) is a mucosal adjuvant capable of inducing strong immune responses to co-administered antigens following oral or intranasal immunization of mice. To date, the direct effect of CT on antigen-specific CD4(+) T cell migration and proliferation profiles in vivo is not well characterized. In this study, the effect of CT on the migration pattern and proliferative responses of adoptively transferred, CD4(+) TCR transgenic T cells in orally or intranasally vaccinated mice, was analyzed by flow cytometry. GFP-expressing or CFSE-labeled OT-II lymphocytes were adoptively transferred to naïve C57BL/6 mice, and mice were subsequently vaccinated with OVA with or without CT via the oral or intranasal route. CT did not alter the migration pattern of antigen-specific T cells, regardless of the route of immunization, but increased the number of transgenic CD4(+) T cells in draining lymphoid tissue. This increase in the number of transgenic CD4(+) T cells was not due to cells undergoing more rounds of cellular division in vivo, suggesting that CT may exert an indirect adjuvant effect on CD4(+) T cells. The findings reported here suggest that CT functions as a mucosal adjuvant by increasing the number of antigen specific CD4(+) T cells independent of their migration pattern or kinetics of cellular division.
Conflict of interest statement
Figures


Similar articles
-
Modulating dendritic cells to optimize mucosal immunization protocols.J Immunol. 1999 Oct 1;163(7):3668-75. J Immunol. 1999. PMID: 10490961
-
Sphingosine-1-phosphate receptor agonism impairs the efficiency of the local immune response by altering trafficking of naive and antigen-activated CD4+ T cells.J Immunol. 2003 Apr 1;170(7):3662-70. doi: 10.4049/jimmunol.170.7.3662. J Immunol. 2003. PMID: 12646631
-
Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues.J Exp Med. 1993 Oct 1;178(4):1309-20. doi: 10.1084/jem.178.4.1309. J Exp Med. 1993. PMID: 8376936 Free PMC article.
-
CD11c(high )dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization.J Immunol. 2009 Oct 15;183(8):5032-41. doi: 10.4049/jimmunol.0803992. Epub 2009 Sep 28. J Immunol. 2009. PMID: 19786541
-
Effects of the adjuvant cholera toxin on dendritic cells: stimulatory and inhibitory signals that result in the amplification of immune responses.Int J Med Microbiol. 2002 Feb;291(6-7):571-5. doi: 10.1078/1438-4221-00169. Int J Med Microbiol. 2002. PMID: 11892684 Review.
References
-
- Northrup RS, Fauci AS (1972) Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. J Infect Dis. 125: 672–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

