A novel rodent model of posterior ischemic optic neuropathy
- PMID: 23544206
- PMCID: PMC4885914
- DOI: 10.1001/2013.jamaophthalmol.271
A novel rodent model of posterior ischemic optic neuropathy
Abstract
Objectives: To develop a reliable, reproducible rat model of posterior ischemic optic neuropathy (PION) and study the cellular responses in the optic nerve and retina.
Methods: Posterior ischemic optic neuropathy was induced in adult rats by photochemically induced ischemia. Retinal and optic nerve vasculature was examined by fluorescein isothiocyanate–dextran extravasation. Tissue sectioning and immunohistochemistry were used to investigate the pathologic changes. Retinal ganglion cell survival at different times after PION induction, with or without neurotrophic application, was quantified by fluorogold retrograde labeling.
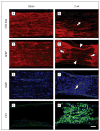
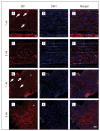
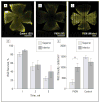
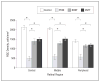
Results: Optic nerve injury was confirmed after PION induction, including local vascular leakage, optic nerve edema, and cavernous degeneration. Immunostaining data revealed microglial activation and focal loss of astrocytes, with adjacent astrocytic hypertrophy. Up to 23%, 50%, and 70% retinal ganglion cell loss was observed at 1 week, 2 weeks, and 3 weeks, respectively, after injury compared with a sham control group. Experimental treatment by brain-derived neurotrophic factor and ciliary neurotrophic factor remarkably prevented retinal ganglion cell loss in PION rats. At 3 weeks after injury, more than 40% of retinal ganglion cells were saved by the application of neurotrophic factors.
Conclusions: Rat PION created by photochemically induced ischemia is a reproducible and reliable animal model for mimicking the key features of human PION.
Clinical relevance: The correspondence between the features of this rat PION model to those of human PION makes it an ideal model to study the pathophysiologic course of the disease, most of which remains to be elucidated. Furthermore, it provides an optimal model for testing therapeutic approaches for optic neuropathies.
Figures






References
-
- Rucker JC, Biousse V, Newman NJ. Ischemic optic neuropathies. Curr Opin Neurol. 2004;17(1):27–35. - PubMed
-
- Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis, and management. Eye (Lond) 2004;18(11):1188–1206. - PubMed
-
- Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009;28(1):34–62. - PubMed
-
- Hayreh SS. Inter-individual variation in blood-supply of the optic nerve head: its importance in various ischemic disorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol. 1985;59 (3):217–246. - PubMed
-
- Jacobson DM, Vierkant RA, Belongia EA. Nonarteritic anterior ischemic optic neuropathy: a case-control study of potential risk factors. Arch Ophthalmol. 1997;115(11):1403–1407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

