Nitroxyl accelerates the oxidation of oxyhemoglobin by nitrite
- PMID: 23545404
- PMCID: PMC3652897
- DOI: 10.1016/j.niox.2013.03.006
Nitroxyl accelerates the oxidation of oxyhemoglobin by nitrite
Abstract
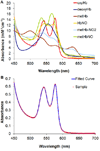
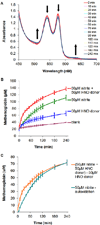
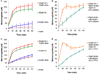
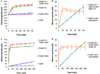
Angeli's salt (Na₂N₂O₃) decomposes into nitroxyl (HNO) and nitrite (NO₂(-)), compounds of physiological and therapeutic interest for their impact on biological signaling both through nitric oxide and nitric oxide independent pathways. Both nitrite and HNO oxidize oxygenated hemoglobin to methemoglobin. Earlier work has shown that HNO catalyzes the reduction of nitrite by deoxygenated hemoglobin. In this work, we have shown that HNO accelerates the oxidation of oxygenated hemoglobin by NO₂(-). We have demonstrated this HNO mediated acceleration of the nitrite/oxygenated hemoglobin reaction with oxygenated hemoglobin being in excess to HNO and nitrite (as would be found under physiological conditions) by monitoring the formation of methemoglobin in the presence of Angeli's salt with and without added NO₂(-). In addition, this acceleration has been demonstrated using the HNO donor 4-nitrosotetrahydro-2H-pyran-4-yl pivalate, a water-soluble acyloxy nitroso compound that does not release NO₂(-) but generates HNO in the presence of esterase. This HNO donor was used both with and without NO₂(-) and acceleration of the NO₂(-) induced formation of methemoglobin was observed. We found that the acceleration was not substantially affected by catalase, superoxide dismutase, c-PTIO, or IHP, suggesting that it is not due to formation of extramolecular peroxide, NO₂ or H₂O₂, or to modulation of allosteric properties. In addition, we found that the acceleration is not likely to be related to HNO binding to free reduced hemoglobin, as we found HNO binding to reduced hemoglobin to be much weaker than has previously been proposed. We suggest that the mechanism of the acceleration involves local propagation of autocatalysis in the nitrite-oxygenated Hb reaction. This acceleration of the nitrite oxyhemoglobin reaction could affect studies aimed at understanding physiological roles of HNO and perhaps nitrite and use of these agents in therapeutics such as hemolytic anemias, heart failure, and ischemia reperfusion injury.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



References
-
- Bermejo E, Saenz DA, Alberto F, Rosenstein RE, Bari SE, Lazzari MA. Effect of nitroxyl on human platelets function. Thrombosis and Haemostasis. 2005;94:578–584. - PubMed
-
- Chazotte-Aubert L, Oikawa S, Gilibert I, Bianchini F, Kawanishi S, Ohshima H. Cytotoxicity and site-specific DNA damage induced by nitroxyl anion (NO−) in the presence of hydrogen peroxide - Implications for various pathophysiological conditions. Journal of Biological Chemistry. 1999;274:20909–20915. - PubMed
-
- Cheong E, Tumbev V, Abramson J, Salama G, Stoyanovsky DA. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium. 2005;37:87–96. - PubMed
-
- Colton CA, Gbadegesin M, Wink DA, Miranda KM, Espey MG, Vicini S. Nitroxyl anion regulation of the NMDA receptor. Journal of Neurochemistry. 2001;78:1126–1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

