Base excision repair
- PMID: 23545420
- PMCID: PMC3683898
- DOI: 10.1101/cshperspect.a012583
Base excision repair
Abstract
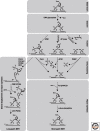
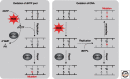
Base excision repair (BER) corrects DNA damage from oxidation, deamination and alkylation. Such base lesions cause little distortion to the DNA helix structure. BER is initiated by a DNA glycosylase that recognizes and removes the damaged base, leaving an abasic site that is further processed by short-patch repair or long-patch repair that largely uses different proteins to complete BER. At least 11 distinct mammalian DNA glycosylases are known, each recognizing a few related lesions, frequently with some overlap in specificities. Impressively, the damaged bases are rapidly identified in a vast excess of normal bases, without a supply of energy. BER protects against cancer, aging, and neurodegeneration and takes place both in nuclei and mitochondria. More recently, an important role of uracil-DNA glycosylase UNG2 in adaptive immunity was revealed. Furthermore, other DNA glycosylases may have important roles in epigenetics, thus expanding the repertoire of BER proteins.
Figures


References
-
- Aamodt RM, Falnes PO, Johansen RF, Seeberg E, Bjoras M 2004. The Bacillus subtilis counterpart of the mammalian 3-methyladenine DNA glycosylase has hypoxanthine and 1,N6-ethenoadenine as preferred substrates. J Biol Chem 279: 13601–13606 - PubMed
-
- Aburatani H, Hippo Y, Ishida T, Takashima R, Matsuba C, Kodama T, Takao M, Yasui A, Yamamoto K, Asano M 1997. Cloning and characterization of mammalian 8-hydroxyguanine-specific DNA glycosylase/apurinic, apyrimidinic lyase, a functional mutM homologue. Cancer Res 57: 2151–2156 - PubMed
-
- Akbari M, Otterlei M, Pena-Diaz J, Aas PA, Kavli B, Liabakk NB, Hagen L, Imai K, Durandy A, Slupphaug G, et al. 2004. Repair of U/G and U/A in DNA by UNG2-associated repair complexes takes place predominantly by short-patch repair both in proliferating and growth-arrested cells. Nucleic Acids Res 32: 5486–5498 - PMC - PubMed
-
- Akbari M, Otterlei M, Pena-Diaz J, Krokan HE 2007. Different organization of base excision repair of uracil in DNA in nuclei and mitochondria and selective upregulation of mitochondrial uracil-DNA glycosylase after oxidative stress. Neuroscience 145: 1201–1212 - PubMed
-
- Akbari M, Visnes T, Krokan HE, Otterlei M 2008. Mitochondrial base excision repair of uracil and AP sites takes place by single-nucleotide insertion and long-patch DNA synthesis. DNA Repair (Amst) 7: 605–616 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
