Postreplicative mismatch repair
- PMID: 23545421
- PMCID: PMC3683899
- DOI: 10.1101/cshperspect.a012633
Postreplicative mismatch repair
Abstract
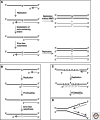
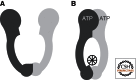
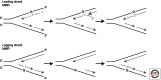
The mismatch repair (MMR) system detects non-Watson-Crick base pairs and strand misalignments arising during DNA replication and mediates their removal by catalyzing excision of the mispair-containing tract of nascent DNA and its error-free resynthesis. In this way, MMR improves the fidelity of replication by several orders of magnitude. It also addresses mispairs and strand misalignments arising during recombination and prevents synapses between nonidentical DNA sequences. Unsurprisingly, MMR malfunction brings about genomic instability that leads to cancer in mammals. But MMR proteins have recently been implicated also in other processes of DNA metabolism, such as DNA damage signaling, antibody diversification, and repair of interstrand cross-links and oxidative DNA damage, in which their functions remain to be elucidated. This article reviews the progress in our understanding of the mechanism of replication error repair made during the past decade.
Figures




References
-
- Acharya S, Foster PL, Brooks P, Fishel R 2003. The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Mol Cell 12: 233–246 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
