Sphingolipid homeostasis in the endoplasmic reticulum and beyond
- PMID: 23545423
- PMCID: PMC3683901
- DOI: 10.1101/cshperspect.a013326
Sphingolipid homeostasis in the endoplasmic reticulum and beyond
Abstract
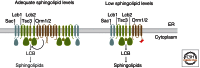
Sphingolipids are a diverse group of lipids that have essential cellular roles as structural components of membranes and as potent signaling molecules. In recent years, a detailed picture has emerged of the basic biochemistry of sphingolipids-from their initial synthesis in the endoplasmic reticulum (ER), to their elaboration into complex glycosphingolipids, to their turnover and degradation. However, our understanding of how sphingolipid metabolism is regulated in response to metabolic demand and physiologic cues remains incomplete. Here I discuss new insights into the mechanisms that ensure sphingolipid homeostasis, with an emphasis on the ER as a critical regulatory site in sphingolipid metabolism. In particular, Orm family proteins have recently emerged as key ER-localized mediators of sphingolipid homeostasis. A detailed understanding of how cells sense and control sphingolipid production promises to provide key insights into membrane function in health and disease.
Figures



References
-
- Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R 2012. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol 14: 542–547 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
