Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors
- PMID: 23545534
- PMCID: PMC3716163
- DOI: 10.1128/AAC.00235-13
Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors
Abstract
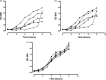
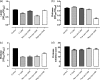
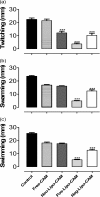
We investigated the efficacy and safety of liposomal clarithromycin formulations with different surface charges against clinical isolates of Pseudomonas aeruginosa from the lungs of cystic fibrosis (CF) patients. The liposomal clarithromycin formulations were prepared by the dehydration-rehydration method, and their sizes were measured using the dynamic-light-scattering technique. Encapsulation efficiency was determined by microbiological assay, and the stabilities of the formulations in biological fluid were evaluated for a period of 48 h. The MICs and minimum bactericidal concentrations (MBCs) of free and liposomal formulations were determined with P. aeruginosa strains isolated from CF patients. Liposomal clarithromycin activity against biofilm-forming P. aeruginosa was compared to that of free antibiotic using the Calgary Biofilm Device (CBD). The effects of subinhibitory concentrations of free and liposomal clarithromycin on bacterial virulence factors and motility on agar were investigated on clinical isolates of P. aeruginosa. The cytotoxicities of the liposome preparations and free drug were evaluated on a pulmonary epithelial cell line (A549). The average diameter of the formulations was >222 nm, with encapsulation efficiencies ranging from 5.7% to 30.4%. The liposomes retained more than 70% of their drug content during the 48-h time period. The highly resistant strains of P. aeruginosa became susceptible to liposome-encapsulated clarithromycin (MIC, 256 mg/liter versus 8 mg/liter; P < 0.001). Liposomal clarithromycin reduced the bacterial growth within the biofilm by 3 to 4 log units (P < 0.001), significantly attenuated virulence factor production, and reduced bacterial twitching, swarming, and swimming motilities. The clarithromycin-entrapped liposomes were less cytotoxic than the free drug (P < 0.001). These data indicate that our novel formulations could be a useful strategy to enhance the efficacy of clarithromycin against resistant P. aeruginosa strains that commonly affect individuals with cystic fibrosis.
Figures

References
-
- Uppaluri L, England SJ, Scanlin TF. 2012. Clinical evidence that V456A is a cystic fibrosis causing mutation in South Asians. J. Cyst. Fibros. 11:312–315 - PubMed
-
- Munder A, Wolbeling F, Kerber-Momot T, Wedekind D, Baumann U, Gulbins E, Tummler B. 2011. Acute intratracheal Pseudomonas aeruginosa infection in cystic fibrosis mice is age-independent. Respir. Res. 12:148 doi:110.1186/1465-9921-1112-1148 - PMC - PubMed
-
- Rogers GB, Hoffman LR, Doring G. 2011. Novel concepts in evaluating antimicrobial therapy for bacterial lung infections in patients with cystic fibrosis. J. Cyst. Fibros. 10:387–400 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

