Balancing the risks and benefits of oxygen therapy in critically III adults
- PMID: 23546490
- PMCID: PMC3616683
- DOI: 10.1378/chest.12-1215
Balancing the risks and benefits of oxygen therapy in critically III adults
Abstract
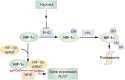
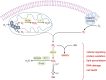
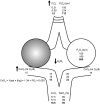
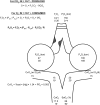
Oxygen therapy is an integral part of the treatment of critically ill patients. Maintenance of adequate oxygen delivery to vital organs often requires the administration of supplemental oxygen, sometimes at high concentrations. Although oxygen therapy is lifesaving, it may be associated with deleterious effects when administered for prolonged periods at high concentrations. Here, we review the recent advances in our understanding of the molecular responses to hypoxia and high levels of oxygen and review the current guidelines for oxygen therapy in critically ill patients.
Figures
References
-
- Priestley J. The Discovery of Oxygen, Part 1. Experiments by Joseph Priestly, LL.D. (1775). Chicago, IL: Albembic Club Reprints: University of Chicago Press; 1912:53-54
-
- Bean JW. Effects of oxygen at increased pressure. Physiol Rev. 1945;25(1):1-147
-
- Becker-Freyseng H, Clamann HG. On the question of oxygen poisoning. J Mol Med. 1939;18(43):1382-1385
-
- Caldwell PR, Lee WL, Jr, Schildkraut HS, Archibald ER. Changes in lung volume, diffusing capacity, and blood gases in men breathing oxygen. J Appl Physiol. 1966;21(5):1477-1483 - PubMed
-
- O’Driscoll BR, Howard LS, Davison AG; British Thoracic Society BTS guideline for emergency oxygen use in adult patients. Thorax. 2008;63(suppl 6):vi1-vi68 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

