The history of Lynch syndrome
- PMID: 23546821
- PMCID: PMC3720817
- DOI: 10.1007/s10689-013-9637-8
The history of Lynch syndrome
Conflict of interest statement
The authors have no conflicts of interest to report
Figures










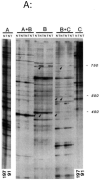

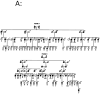















References
-
- Warthin AS. Heredity with reference to carcinoma as shown by the study of the cases examined in the Pathological Laboratory of the University of Michigan, 1895-1912. Arch Int Med. 1913;12:546–555.
-
- Warthin AS. The further study of a cancer family. J Cancer Research. 1925;9:279–286.
-
- Hauser IJ, Weller CV. A further report on the cancer family of Warthin. American Journal of Cancer. 1936;27:434–449.
-
- Bargen JA, Mayo CW, Giffin LA. Familial trends in human cancer. J Heredity. 1941;32:7.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

