Aneuploidy in health, disease, and aging
- PMID: 23547028
- PMCID: PMC3613689
- DOI: 10.1083/jcb.201301061
Aneuploidy in health, disease, and aging
Abstract
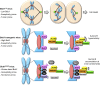
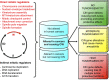
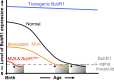
Aneuploidy, an aberrant number of chromosomes, has been recognized as a feature of human malignancies for over a century, but compelling evidence for causality was largely lacking until mouse models for chromosome number instability were used. These in vivo studies have not only uncovered important new insights into the extremely complex aneuploidy-cancer relationship but also into the molecular mechanisms underlying proper and aberrant chromosome segregation. A series of diverse mouse models for the mitotic checkpoint protein BubR1 has provided evidence for a provocative novel link between aneuploidization and the development of age-related pathologies.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

