Sex-biased gene expression at homomorphic sex chromosomes in emus and its implication for sex chromosome evolution
- PMID: 23547111
- PMCID: PMC3631621
- DOI: 10.1073/pnas.1217027110
Sex-biased gene expression at homomorphic sex chromosomes in emus and its implication for sex chromosome evolution
Abstract
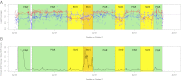
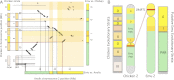
Sex chromosomes originate from autosomes. The accumulation of sexually antagonistic mutations on protosex chromosomes selects for a loss of recombination and sets in motion the evolutionary processes generating heteromorphic sex chromosomes. Recombination suppression and differentiation are generally viewed as the default path of sex chromosome evolution, and the occurrence of old, homomorphic sex chromosomes, such as those of ratite birds, has remained a mystery. Here, we analyze the genome and transcriptome of emu (Dromaius novaehollandiae) and confirm that most genes on the sex chromosome are shared between the Z and W. Surprisingly, however, levels of gene expression are generally sex-biased for all sex-linked genes relative to autosomes, including those in the pseudoautosomal region, and the male-bias increases after gonad formation. This expression bias suggests that the emu sex chromosomes have become masculinized, even in the absence of ZW differentiation. Thus, birds may have taken different evolutionary solutions to minimize the deleterious effects imposed by sexually antagonistic mutations: some lineages eliminate recombination along the protosex chromosomes to physically restrict sexually antagonistic alleles to one sex, whereas ratites evolved sex-biased expression to confine the product of a sexually antagonistic allele to the sex it benefits. This difference in conflict resolution may explain the preservation of recombining, homomorphic sex chromosomes in other lineages and illustrates the importance of sexually antagonistic mutations driving the evolution of sex chromosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bergero R, Charlesworth D. The evolution of restricted recombination in sex chromosomes. Trends Ecol Evol. 2009;24(2):94–102. - PubMed
-
- Bull JJ. Evolution of Sex Determining Mechanisms. Menlo Park, CA: Benjamin/Cummings; 1983.
-
- Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity (Edinb) 2005;95(2):118–128. - PubMed
-
- Rice WR. Sex-chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38(4):735–742. - PubMed
-
- Rice WR. The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex chromosomes. Evolution. 1987;41(4):911–914. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

