DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae
- PMID: 23547164
- PMCID: PMC3606085
- DOI: 10.1534/genetics.112.145219
DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae
Abstract
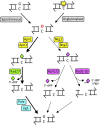
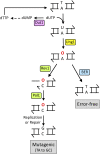
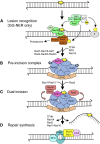
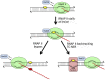
DNA repair mechanisms are critical for maintaining the integrity of genomic DNA, and their loss is associated with cancer predisposition syndromes. Studies in Saccharomyces cerevisiae have played a central role in elucidating the highly conserved mechanisms that promote eukaryotic genome stability. This review will focus on repair mechanisms that involve excision of a single strand from duplex DNA with the intact, complementary strand serving as a template to fill the resulting gap. These mechanisms are of two general types: those that remove damage from DNA and those that repair errors made during DNA synthesis. The major DNA-damage repair pathways are base excision repair and nucleotide excision repair, which, in the most simple terms, are distinguished by the extent of single-strand DNA removed together with the lesion. Mistakes made by DNA polymerases are corrected by the mismatch repair pathway, which also corrects mismatches generated when single strands of non-identical duplexes are exchanged during homologous recombination. In addition to the true repair pathways, the postreplication repair pathway allows lesions or structural aberrations that block replicative DNA polymerases to be tolerated. There are two bypass mechanisms: an error-free mechanism that involves a switch to an undamaged template for synthesis past the lesion and an error-prone mechanism that utilizes specialized translesion synthesis DNA polymerases to directly synthesize DNA across the lesion. A high level of functional redundancy exists among the pathways that deal with lesions, which minimizes the detrimental effects of endogenous and exogenous DNA damage.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

