Targeting both IGF-1R and mTOR synergistically inhibits growth of renal cell carcinoma in vitro
- PMID: 23548153
- PMCID: PMC3623828
- DOI: 10.1186/1471-2407-13-170
Targeting both IGF-1R and mTOR synergistically inhibits growth of renal cell carcinoma in vitro
Abstract
Background: Advanced or metastatic renal cell carcinoma (RCC) has a poor prognosis, because it is relatively resistant to conventional chemotherapy or radiotherapy. Treatments with human interferon-α2b alone or in combination with mammalian target of rapamycin (mTOR) inhibitors have led to only a modest improvement in clinical outcome. One observation made with mTOR inhibitors is that carcinomas can overcome these inhibitory effects by activating the insulin-like growth factor-I (IGF-I) signaling pathway. Clinically, there is an association of IGF-I receptor (IGF-IR) expression in RCC and poor long-term patient survival. We have developed a humanized anti-IGF-IR monoclonal antibody, hR1, which binds to RCC, resulting in effective down-regulation of IGF-IR and moderate inhibition of cell proliferation in vitro. In this work, we evaluate the anti-tumor activity of two novel IGF-1R-targeting agents against renal cell carcinoma given alone or in combination with an mTOR inhibitor.
Methods: hR1 was linked by the DOCK-AND-LOCK™ (DNL™) method to four Fabs of hR1, generating Hex-hR1, or to four molecules of interferon-α2b, generating 1R-2b. Eight human RCC cell lines were screened for IGF-1R expression and sensitivity to treatment with hR1 in vitro. Synergy with an mTOR inhibitor, temsirolimus, was tested in a cell line (ACHN) with low sensitivity to hR1.
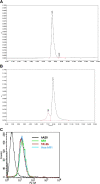
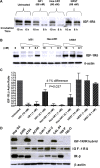
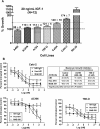
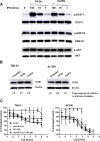
Results: Hex-hR1 induced the down-regulation of IGF-IR at 10-fold lower concentrations compared to the parental hR1. Sensitivity to growth inhibition mediated by hR1 and Hex-hR1 treatments correlated with IGF-1R expression (higher expression was more sensitive). The potency of 1R-2b to inhibit the in vitro growth of RCC was also demonstrated in two human cell lines, ACHN and 786-O, with EC50-values of 63 and 48 pM, respectively. When combined with temsirolimus, a synergistic growth-inhibition with hR1, Hex-hR1, and 1R-2b was observed in ACHN cells at concentrations as low as 10 nM for hR1, 1 nM for Hex-hR1, and 2.6 nM for 1R-2b.
Conclusions: Both Hex-hR1 and 1R-2b proved to be more potent than parental hR1 in inhibiting growth of RCC in vitro. Synergy was achieved when each of the three hR1-based agents was combined with temsirolimus, suggesting a new approach for treating RCC.
Figures

References
-
- American Cancer Society. Cancer Facts and Figures 2012. Atlanta: American Cancer Society; 2012.
-
- Aschenbrenner DS. New drug approved for advanced renal cell carcinoma. Am J Nurs. 2012;112:22–23. - PubMed
-
- Gollob JA, Rathmell WK, Richmond TM, Marino CB, Miller EK, Grigson G, Watkins C, Gu L, Peterson BL, Wright JJ. Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol. 2007;25:3288–3295. doi: 10.1200/JCO.2007.10.8613. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

