High-fat diet feeding causes rapid, non-apoptotic cleavage of caspase-3 in astrocytes
- PMID: 23548599
- PMCID: PMC3684737
- DOI: 10.1016/j.brainres.2013.03.033
High-fat diet feeding causes rapid, non-apoptotic cleavage of caspase-3 in astrocytes
Abstract
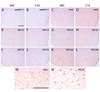
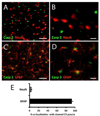
Astrocytes respond to multiple forms of central nervous system (CNS) injury by entering a reactive state characterized by morphological changes and a specific pattern of altered protein expression. Termed astrogliosis, this response has been shown to strongly influence the injury response and functional recovery of CNS tissues. This pattern of CNS inflammation and injury associated with astrogliosis has recently been found to occur in the energy homeostasis centers of the hypothalamus during diet-induced obesity (DIO) in rodent models, but the characterization of the astrocyte response remains incomplete. Here, we report that astrocytes in the mediobasal hypothalamus respond robustly and rapidly to purified high-fat diet (HFD) feeding by cleaving caspase-3, a protease whose cleavage is often associated with apoptosis. Although obesity develops in HFD-fed rats by day 14, caspase-3 cleavage occurs by day 3, prior to the development of obesity, suggesting the possibility that it could play a causal role in the hypothalamic neuropathology and fat gain observed in DIO. Caspase-3 cleavage is not associated with an increase in the rate of apoptosis, as determined by TUNEL staining, suggesting it plays a non-apoptotic role analogous to the response to excitotoxic neuron injury. Our results indicate that astrocytes in the mediobasal hypothalamus respond rapidly and robustly to HFD feeding, activating caspase-3 in the absence of apoptosis, a process that has the potential to influence the course of DIO.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures



References
-
- Acarin L, et al. Astroglial nitration after postnatal excitotoxic damage: correlation with nitric oxide sources, cytoskeletal, apoptotic and antioxidant proteins. J Neurotrauma. 2005;22:189–200. - PubMed
-
- Acarin L, et al. Caspase-3 activation in astrocytes following postnatal excitotoxic damage correlates with cytoskeletal remodeling but not with cell death or proliferation. Glia. 2007;55:954–965. - PubMed
-
- Beer R, et al. Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J Neurochem. 2000;75:1264–1273. - PubMed
-
- De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007028/HL/NHLBI NIH HHS/United States
- T32 DK007247/DK/NIDDK NIH HHS/United States
- R01 DK090320/DK/NIDDK NIH HHS/United States
- DK017047/DK/NIDDK NIH HHS/United States
- F32DK091989/DK/NIDDK NIH HHS/United States
- R01 DK083042/DK/NIDDK NIH HHS/United States
- DK052989/DK/NIDDK NIH HHS/United States
- P01 DK068384/DK/NIDDK NIH HHS/United States
- DK083042/DK/NIDDK NIH HHS/United States
- T32DK007247/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- R01 DK052989/DK/NIDDK NIH HHS/United States
- K08 DK088872/DK/NIDDK NIH HHS/United States
- DK088872/DK/NIDDK NIH HHS/United States
- P30DK017047/DK/NIDDK NIH HHS/United States
- P30 DK035816/DK/NIDDK NIH HHS/United States
- F32 DK091989/DK/NIDDK NIH HHS/United States
- DK068384/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

