Pseudomonas aeruginosa small protease (PASP), a keratitis virulence factor
- PMID: 23548618
- PMCID: PMC3632270
- DOI: 10.1167/iovs.13-11788
Pseudomonas aeruginosa small protease (PASP), a keratitis virulence factor
Abstract
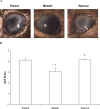
Purpose: The virulence contribution of Pseudomonas aeruginosa small protease (PASP) during experimental keratitis was studied by comparing a PASP-deficient mutant with its parent and rescue strains.
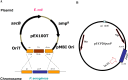
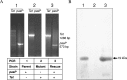
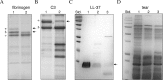
Methods: The pasP gene of P. aeruginosa was replaced with the tetracycline resistance gene via allelic exchange. A plasmid carrying the pasP gene was introduced into the PASP-deficient mutant to construct a rescue strain. The PASP protein in the culture supernatants was determined by Western blot analysis. Corneal virulence was evaluated in rabbit and mouse keratitis models by slit lamp examination (SLE), bacterial enumeration, and/or histopathological analysis. Various host proteins and the rabbit tear film were analyzed for their susceptibility to PASP degradation.
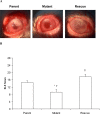
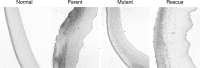
Results: The PASP-deficient mutant produced a significantly lower mean SLE score when compared with the parent or rescue strain (P ≤ 0.03) at 29 hours postinfection (PI). All of the strains grew equally in the rabbit cornea (P = 0.971). Corneas infected with the PASP-deficient mutant showed moderate histopathology compared with those infected with the parent or rescue strain, which produced severe pathology inclusive of epithelial erosions, corneal edema, and neutrophil infiltration. In the mouse model, eyes inoculated with the PASP-deficient mutant had a significantly lower mean SLE score at 24 hours PI than the eyes inoculated with the parent or rescue strain (P ≤ 0.007). PASP was found to degrade complement C3, fibrinogen, antimicrobial peptide LL-37, and constituents of the tear film.
Conclusions: PASP is a commonly secreted protease of P. aeruginosa that contributes significantly to the pathogenesis of keratitis.
Figures
Similar articles
-
Mechanism of Pseudomonas aeruginosa Small Protease (PASP), a Corneal Virulence Factor.Invest Ophthalmol Vis Sci. 2018 Dec 3;59(15):5993-6002. doi: 10.1167/iovs.18-25834. Invest Ophthalmol Vis Sci. 2018. PMID: 30572344 Free PMC article.
-
Alkaline protease-deficient mutants of Pseudomonas aeruginosa are virulent in the eye.Curr Eye Res. 2000 Sep;21(3):730-9. Curr Eye Res. 2000. PMID: 11120561
-
Pseudomonas aeruginosa LasA protease and corneal infections.Curr Eye Res. 2001 Apr;22(4):266-71. doi: 10.1076/ceyr.22.4.266.5509. Curr Eye Res. 2001. PMID: 11462165
-
Pseudomonas aeruginosa Keratitis: Protease IV and PASP as Corneal Virulence Mediators.Microorganisms. 2019 Aug 22;7(9):281. doi: 10.3390/microorganisms7090281. Microorganisms. 2019. PMID: 31443433 Free PMC article. Review.
-
Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist.Microbes Infect. 2000 Jul;2(9):1051-60. doi: 10.1016/s1286-4579(00)01259-4. Microbes Infect. 2000. PMID: 10967285 Review.
Cited by
-
Phage Therapy Is Effective in a Mouse Model of Bacterial Equine Keratitis.Appl Environ Microbiol. 2016 Aug 15;82(17):5332-9. doi: 10.1128/AEM.01166-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27342558 Free PMC article.
-
Post-secretional activation of Protease IV by quorum sensing in Pseudomonas aeruginosa.Sci Rep. 2017 Jun 30;7(1):4416. doi: 10.1038/s41598-017-03733-6. Sci Rep. 2017. PMID: 28667333 Free PMC article.
-
Infectious keratitis: secreted bacterial proteins that mediate corneal damage.J Ophthalmol. 2013;2013:369094. doi: 10.1155/2013/369094. Epub 2013 Jan 8. J Ophthalmol. 2013. PMID: 23365719 Free PMC article.
-
Functional expression, purification, and biochemical properties of subtilase SprP from Pseudomonas aeruginosa.Microbiologyopen. 2015 Oct;4(5):743-52. doi: 10.1002/mbo3.275. Epub 2015 Jul 14. Microbiologyopen. 2015. PMID: 26175208 Free PMC article.
-
Comparison of clinical characteristics and antibiotic susceptibility between Pseudomonas aeruginosa and P. putida keratitis at a tertiary referral center: a retrospective study.BMC Ophthalmol. 2018 Aug 20;18(1):204. doi: 10.1186/s12886-018-0882-3. BMC Ophthalmol. 2018. PMID: 30126384 Free PMC article.
References
-
- Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008; 27: 22–27 - PubMed
-
- Giese MJ, Weissman BA. Contact lens associated corneal infections. Where do we go from here? Clin Exp Optom. 2002; 85: 141–148 - PubMed
-
- Mah-Sadorra JH, Yavuz SG, Najjar DM, Laibson PR, Rapuano CJ, Cohen EJ. Trends in contact lens-related corneal ulcers. Cornea. 2005; 24: 51–58 - PubMed
-
- Kessler E, Kennah HE, Brown SI. Pseudomonas protease. Purification, partial characterization, and its effect on collagen, proteoglycan, and rabbit corneas. Invest Ophthalmol Vis Sci. 1977; 16: 488–497 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

