Mycolactone activation of Wiskott-Aldrich syndrome proteins underpins Buruli ulcer formation
- PMID: 23549080
- PMCID: PMC3613928
- DOI: 10.1172/JCI66576
Mycolactone activation of Wiskott-Aldrich syndrome proteins underpins Buruli ulcer formation
Abstract
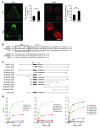
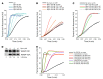
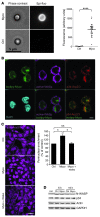
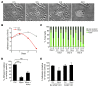
Mycolactone is a diffusible lipid secreted by the human pathogen Mycobacterium ulcerans, which induces the formation of open skin lesions referred to as Buruli ulcers. Here, we show that mycolactone operates by hijacking the Wiskott-Aldrich syndrome protein (WASP) family of actin-nucleating factors. By disrupting WASP autoinhibition, mycolactone leads to uncontrolled activation of ARP2/3-mediated assembly of actin in the cytoplasm. In epithelial cells, mycolactone-induced stimulation of ARP2/3 concentrated in the perinuclear region, resulting in defective cell adhesion and directional migration. In vivo injection of mycolactone into mouse ears consistently altered the junctional organization and stratification of keratinocytes, leading to epidermal thinning, followed by rupture. This degradation process was efficiently suppressed by coadministration of the N-WASP inhibitor wiskostatin. These results elucidate the molecular basis of mycolactone activity and provide a mechanism for Buruli ulcer pathogenesis. Our findings should allow for the rationale design of competitive inhibitors of mycolactone binding to N-WASP, with anti-Buruli ulcer therapeutic potential.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

