Hepatic glucose sensing is required to preserve β cell glucose competence
- PMID: 23549084
- PMCID: PMC3613916
- DOI: 10.1172/JCI65538
Hepatic glucose sensing is required to preserve β cell glucose competence
Abstract
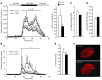
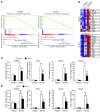
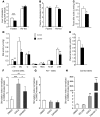
Liver glucose metabolism plays a central role in glucose homeostasis and may also regulate feeding and energy expenditure. Here we assessed the impact of glucose transporter 2 (Glut2) gene inactivation in adult mouse liver (LG2KO mice). Loss of Glut2 suppressed hepatic glucose uptake but not glucose output. In the fasted state, expression of carbohydrate-responsive element-binding protein (ChREBP) and its glycolytic and lipogenic target genes was abnormally elevated. Feeding, energy expenditure, and insulin sensitivity were identical in LG2KO and control mice. Glucose tolerance was initially normal after Glut2 inactivation, but LG2KO mice exhibited progressive impairment of glucose-stimulated insulin secretion even though β cell mass and insulin content remained normal. Liver transcript profiling revealed a coordinated downregulation of cholesterol biosynthesis genes in LG2KO mice that was associated with reduced hepatic cholesterol in fasted mice and reduced bile acids (BAs) in feces, with a similar trend in plasma. We showed that chronic BAs or farnesoid X receptor (FXR) agonist treatment of primary islets increases glucose-stimulated insulin secretion, an effect not seen in islets from Fxr(-/-) mice. Collectively, our data show that glucose sensing by the liver controls β cell glucose competence and suggest BAs as a potential mechanistic link.
Figures
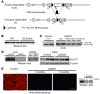

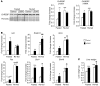
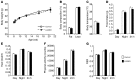
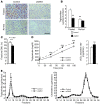

References
-
- Burcelin R, del Carmen Muñoz M, Guillam MT, Thorens B. Liver hyperplasia and paradoxical regulation of glycogen metabolism and glucose-sensitive gene expression in GLUT2-null hepatocytes. Further evidence for the existence of a membrane-based glucose release pathway. J Biol Chem. 2000;275(15):10930–10936. doi: 10.1074/jbc.275.15.10930. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

