Peripheral reproductive organ health and melatonin: ready for prime time
- PMID: 23549263
- PMCID: PMC3645684
- DOI: 10.3390/ijms14047231
Peripheral reproductive organ health and melatonin: ready for prime time
Abstract
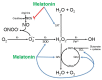
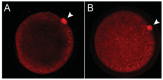
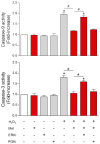
Melatonin has a wide variety of beneficial actions at the level of the gonads and their adnexa. Some actions are mediated via its classic membrane melatonin receptors while others seem to be receptor-independent. This review summarizes many of the published reports which confirm that melatonin, which is produced in the ovary, aids in advancing follicular maturation and preserving the integrity of the ovum prior to and at the time of ovulation. Likewise, when ova are collected for in vitro fertilization-embryo transfer, treating them with melatonin improves implantation and pregnancy rates. Melatonin synthesis as well as its receptors have also been identified in the placenta. In this organ, melatonin seems to be of particular importance for the maintenance of the optimal turnover of cells in the villous trophoblast via its ability to regulate apoptosis. For male gametes, melatonin has also proven useful in protecting them from oxidative damage and preserving their viability. Incubation of ejaculated animal sperm improves their motility and prolongs their viability. For human sperm as well, melatonin is also a valuable agent for protecting them from free radical damage. In general, the direct actions of melatonin on the gonads and adnexa of mammals indicate it is an important agent for maintaining optimal reproductive physiology.
Figures






References
-
- Lerner A.B., Case J.D., Takahashi Y., Lee Y., Mori W. Isolation of melatonin, the pineal factor that lightens melanocytes. J. Am. Chem. Soc. 1958;80:2587.
-
- Launay J.M., Lemaitre B.J., Husson H.P., Dreux C., Hartmann L., da Prada M. Melatonin synthesis by rabbit platelets. Life Sci. 1982;31:1487–1494. - PubMed
-
- Reiter R.J., Richardson B.A., Matthews S.A., Lane S.J., Ferguson B.N. Rhythms of immunoreactive melatonin in the retina and Harderian glands of rats: Persistence after pinealectomy. Life Sci. 1983;32:1299–1236. - PubMed
-
- Huether G., Poeggeler B., Reimer A., George A. Effects of tryptophan administration on circulating melatonin levels in chicks and rats: Evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 1992;51:946–953. - PubMed
-
- Abe M., Itoh M.T., Miyata M., Shimizu K., Sumi Y. Circadian rhythm of serotonin N-acetyltransferace activity in rat lens. Exp. Eye Res. 2000;70:805–808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

