Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease
- PMID: 23550085
- PMCID: PMC3580884
- DOI: 10.1111/jdi.12042
Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease
Abstract
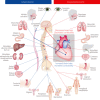
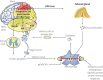
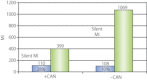
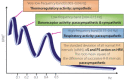
One of the most overlooked of all serious complications of diabetes is cardiovascular autonomic neuropathy. There is now clear evidence that suggests activation of inflammatory cytokines in diabetic patients and that these correlate with abnormalities in sympathovagal balance. Dysfunction of the autonomic system predicts cardiovascular risk and sudden death in patients with type 2 diabetes. It also occurs in prediabetes, providing opportunities for early intervention. Simple tests that can be carried out at the bedside with real-time output of information - within the scope of the practicing physician - facilitate diagnosis and allow the application of sound strategies for management. The window of opportunity for aggressive control of all the traditional risk factors for cardiovascular events or sudden death with intensification of therapy is with short duration diabetes, the absence of cardiovascular disease and a history of severe hypoglycemic events. To this list we can now add autonomic dysfunction and neuropathy, which have become the most powerful predictors of risk for mortality. It seems prudent that practitioners should be encouraged to become familiar with this information and apply risk stratification in clinical practice. Several agents have become available for the correction of functional defects in the autonomic nervous system, and restoration of autonomic balance is now possible.
Keywords: Cardiac autonomic neuropathy; Inflammation; Pathogenesis.
Figures




References
-
- Vinik AI, Maser RE, Mitchell BD, et al Diabetic Autonomic Neuropathy. Diabetes Care 2003; 26: 1553–1579 - PubMed
-
- Maser RE, Mitchell BD, Vinik AI, et al The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta‐analysis. Diabetes Care 2003; 26: 1895–1901 - PubMed
-
- Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation 2007; 115: 387–397 - PubMed
-
- Saravia F, Homo‐Delarche F. Is innervation an early target in autoimmune diabetes? Trends Immunol 2003; 24: 574–579 - PubMed
-
- Borovikova LV, Ivanova S, Zhang M, et al Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000; 405: 458–462 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

