The safety and efficacy of intracoronary nitrite infusion during acute myocardial infarction (NITRITE-AMI): study protocol of a randomised controlled trial
- PMID: 23550096
- PMCID: PMC3641434
- DOI: 10.1136/bmjopen-2013-002813
The safety and efficacy of intracoronary nitrite infusion during acute myocardial infarction (NITRITE-AMI): study protocol of a randomised controlled trial
Abstract
Introduction: Acute myocardial infarction (AMI) is a major cause of death and disability in the UK and worldwide. Presently, timely and effective reperfusion with primary percutaneous coronary intervention (PPCI) remains the most effective treatment strategy for limiting infarct size, preserving left ventricular ejection fraction (LVEF) and improving clinical outcomes. However, the process of reperfusion can itself induce cardiomyocyte death, known as myocardial reperfusion injury, for which there is currently no effective therapy. Extensive preclinical evidence exists to suggest that sodium nitrite (as a source of endogenous nitric oxide) is an effective therapeutic strategy for preventing myocardial reperfusion injury. The purpose of NITRITE-AMI is to test whether sodium nitrite reduces reperfusion injury and subsequent infarct size in patients undergoing PPCI for MI.
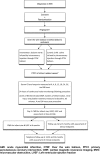
Methods and design: NITRITE-AMI is a double-blind, randomised, single-centre, placebo-controlled trial to determine whether intracoronary nitrite injection reduces infarct size in patients with myocardial infarction undergoing primary angioplasty. The study will enrol 80 patients presenting with ST-elevation myocardial infarction. Patients will be randomised to receive either a bolus of intracoronary sodium nitrite or placebo (sodium chloride) at the time of PPCI. The primary outcome is infarct size assessed by creatine kinase area under the curve (AUC) over 48 h. Secondary endpoints include troponin T AUC and infarct size, LV dimensions and myocardial salvage index assessed by cardiac MR (CMR), markers of platelet reactivity and inflammation, the safety and tolerability of intracoronary nitrite, and 1 year major adverse cardiac events.
Ethics and dissemination: The study is approved by the local ethics committee (NRES Committee London West London: 11/LO/1500) and by the Medicines and Healthcare Products Regulatory Agency (MHRA) (EudraCT nr. 2010-022460-12). The results of the trial will be published according to the CONSORT statement and will be presented at conferences and reported in peer-reviewed journals.
Trial registration: United Kingdom Clinical Research Network (Study ID 12117), http://clinicaltrials.gov (NCT01584453) and Current Controlled Trials (ISRCTN:38736987).
References
-
- Lambert L, Brown K, Segal E, et al. Association between timeliness of reperfusion therapy and clinical outcomes in ST-elevation myocardial infarction. JAMA 2010;303:2148–55 - PubMed
-
- Berger PB, Ellis SG, Holmes DR, Jr, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction. Results from the global use of strategies to open occluded arteries in acute coronary syndromes (GUSTO-IIb) trial. Circulation 1999;100:14–20 - PubMed
-
- McNamara RL, Wang Y, Herrin J, et al. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2006;47:2180–6 - PubMed
-
- Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 2000;283:2941–7 - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical