Assembly of the Synaptonemal Complex Is a Highly Temperature-Sensitive Process That Is Supported by PGL-1 During Caenorhabditis elegans Meiosis
- PMID: 23550120
- PMCID: PMC3618346
- DOI: 10.1534/g3.112.005165
Assembly of the Synaptonemal Complex Is a Highly Temperature-Sensitive Process That Is Supported by PGL-1 During Caenorhabditis elegans Meiosis
Abstract
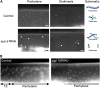
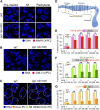
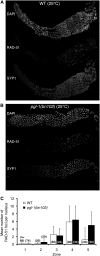
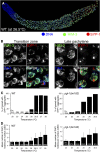
Successful chromosome segregation during meiosis depends on the synaptonemal complex (SC), a structure that stabilizes pairing between aligned homologous chromosomes. Here we show that SC assembly is a temperature-sensitive process during Caenorhabditis elegans meiosis. Temperature sensitivity of SC assembly initially was revealed through identification of the germline-specific P-granule component PGL-1 as a factor promoting stable homolog pairing. Using an assay system that monitors homolog pairing in vivo, we showed that depletion of PGL-1 at 25° disrupts homolog pairing. Analysis of homolog pairing at other chromosomal loci in a pgl-1-null mutant revealed a pairing defect similar to that observed in mutants lacking SC central region components. Furthermore, loss of pgl-1 function at temperatures ≥25° results in severe impairment in loading of SC central region component SYP-1 onto chromosomes, resulting in formation of SYP-1 aggregates. SC assembly is also temperature sensitive in wild-type worms, which exhibit similar SYP-1 loading defects and formation of SYP-1 aggregates at temperatures ≥26.5°. Temperature shift analyses suggest that assembly of the SC is temperature sensitive, but maintenance of the SC is not. We suggest that the temperature sensitive (ts) nature of SC assembly may contribute to fitness and adaptation capacity in C. elegans by enabling meiotic disruption in response to environmental change, thereby increasing the production of male progeny available for outcrossing.
Keywords: P-granule; aneuploid gametes; pachytene; polycomplex; synapsis.
Copyright © 2013 Bilgir et al.
Figures

References
-
- Colaiacovo M. P., MacQueen A. J., Martinez-Perez E., McDonald K., Adamo A., et al. , 2003. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 5: 463–474 - PubMed
-
- Couteau F., Nabeshima K., Villeneuve A., Zetka M., 2004. A component of C. elegans meiotic chromosome axes at the interface of homolog alignment, synapsis, nuclear reorganization, and recombination. Curr. Biol. 14: 585–592 - PubMed
-
- Dombecki C. R., Chiang A. C. Y., Kang H.-J., Bilgir C., Stefanski N. A., et al. , 2011. The Chromodomain protein MRG-1 facilitates SC-independent homologous pairing during meiosis in Caenorhabditis elegans. Dev. Cell 21: 1092–1103 - PubMed
-
- Felix M. A., Braendle C., 2010. The natural history of Caenorhabditis elegans. Curr. Biol. 20: R965–R969 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
