Reproductive status alters transcriptomic response to infection in female Drosophila melanogaster
- PMID: 23550122
- PMCID: PMC3656730
- DOI: 10.1534/g3.112.005306
Reproductive status alters transcriptomic response to infection in female Drosophila melanogaster
Abstract
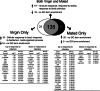
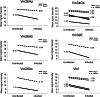
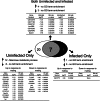
Mating and consequent reproduction significantly reduce the ability of female Drosophila melanogaster to defend against systemic bacterial infection. The goal of the present study was to identify genes likely to inform the mechanism of this post-mating immunosuppression. We used microarrays to contrast genome-wide transcript levels in virgin vs. mated females before and after infection. Because the immunosuppressive effect of mating is contingent on the presence of a germline in females, we repeated the entire experiment by using female mutants that do not form a germline. We found that multiple genes involved in egg production show reduced expression in response to infection, and that this reduction is stronger in virgins than it is in mated females. In germline-less females, expression of egg-production genes was predictably low and not differentially affected by infection. We also identified several immune responsive genes that are differentially induced after infection in virgins vs. mated females. Immune genes affected by mating status and egg production genes altered by infection are candidates to inform the mechanism of the trade-off between mating and immune defense.
Keywords: Drosophila melanogaster; gene expression; immunity; microarray; reproduction.
Figures



References
-
- Agaisse H., Perrimon N., 2004. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 198: 72–82 - PubMed
-
- Agaisse H., Petersen U.-M., Boutros M., Mathey-Prevot B., Perrimon N., 2003. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 5: 441–450 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
