The basic principles of chimeric antigen receptor design
- PMID: 23550147
- PMCID: PMC3667586
- DOI: 10.1158/2159-8290.CD-12-0548
The basic principles of chimeric antigen receptor design
Abstract
Chimeric antigen receptors (CAR) are recombinant receptors that provide both antigen-binding and T-cell-activating functions. A multitude of CARs has been reported over the past decade, targeting an array of cell surface tumor antigens. Their biologic functions have dramatically changed following the introduction of tripartite receptors comprising a costimulatory domain, termed second-generation CARs. These have recently shown clinical benefit in patients treated with CD19-targeted autologous T cells. CARs may be combined with costimulatory ligands, chimeric costimulatory receptors, or cytokines to further enhance T-cell potency, specificity, and safety. CARs represent a new class of drugs with exciting potential for cancer immunotherapy.
Significance: CARs are a new class of drugs with great potential for cancer immunotherapy. Upon their expression in T lymphocytes, CARs direct potent, targeted immune responses that have recently shown encouraging clinical outcomes in a subset of patients with B-cell malignancies. This review focuses on the design of CARs, including the requirements for optimal antigen recognition and different modalities to provide costimulatory support to targeted T cells, which include the use of second- and third generation CARs, costimulatory ligands, chimeric costimulatory receptors, and cytokines.
©2013 AACR.
Conflict of interest statement
I, Michel Sadelain, confirm there is no conflict of interest.
Figures
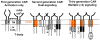

References
-
- Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. - PubMed
-
- Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer cell. 2003;3:431–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

