MicroRNA function in NK-cell biology
- PMID: 23550637
- PMCID: PMC3621029
- DOI: 10.1111/imr.12045
MicroRNA function in NK-cell biology
Abstract
The important role of microRNAs in directing immune responses has become increasingly clear. Here, we highlight discoveries uncovering the role of specific microRNAs in regulating the development and function of natural killer (NK) cells. Furthermore, we discuss the impact of NK cells on the entire immune system during global and specific microRNA ablation in the settings of inflammation, infection, and immune dysregulation.
© 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
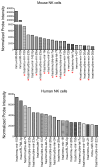
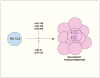

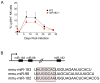

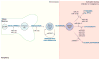
References
-
- Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. - PubMed
-
- Shanley JD. In vivo administration of monoclonal antibody to the NK 1. 1 antigen of natural killer cells: effect on acute murine cytomegalovirus infection. J Med Virol. 1990;30:58–60. - PubMed
-
- Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 2000;45:33–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

