Regulation of immune responses and tolerance: the microRNA perspective
- PMID: 23550642
- PMCID: PMC3684622
- DOI: 10.1111/imr.12060
Regulation of immune responses and tolerance: the microRNA perspective
Abstract
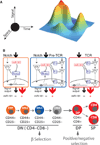
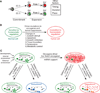
Much has been learned about the molecular and cellular components critical for the control of immune responses and tolerance. It remains a challenge, however, to control the immune response and tolerance at the system level without causing significant toxicity to normal tissues. Recent studies suggest that microRNA (miRNA) genes, an abundant class of non-coding RNA genes that produce characteristic approximately 22 nucleotides small RNAs, play important roles in immune cells. In this article, we discuss emerging knowledge regarding the functions of miRNA genes in the immune system. We delve into the roles of miRNAs in regulating signaling strength and threshold, homeostasis, and the dynamics of the immune response and tolerance during normal and pathogenic immunological conditions. We also present observations based on analyzes of miR-181 family genes that indicate the potential functions of primary and/or precursor miRNAs in target recognition and explore the impact of these findings on target identification. Finally, we illustrate that despite the subtle effects of miRNAs on gene expression, miRNAs have the potential to influence the outcomes of normal and pathogenic immune responses by controlling the quantitative and dynamic aspects of immune responses. Tuning miRNA functions in immune cells, through gain- and loss-of-function approaches in mice, may reveal novel approach to restore immune equilibrium from pathogenic conditions, such as autoimmune disease and leukemia, without significant toxicity.
© 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd.
Figures
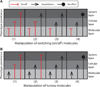
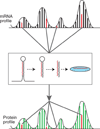
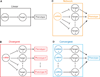
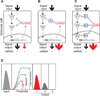

References
-
- Ambros V. The evolution of our thinking about microRNAs. Nat Med. 2008;14:1036–1040. - PubMed
-
- Ruvkun G. The perfect storm of tiny RNAs. Nat Med. 2008;14:1041–1045. - PubMed
-
- Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. - PubMed
-
- Lodish HF, Zhou B, Liu G, Chen CZ. Micromanagement of the immune system by microRNAs. Nat Rev Immunol. 2008;8:120–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

