Nucleus accumbens GABAergic inhibition generates intense eating and fear that resists environmental retuning and needs no local dopamine
- PMID: 23551138
- PMCID: PMC3672387
- DOI: 10.1111/ejn.12194
Nucleus accumbens GABAergic inhibition generates intense eating and fear that resists environmental retuning and needs no local dopamine
Abstract
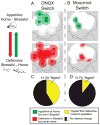
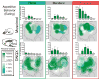
Intense fearful behavior and/or intense appetitive eating behavior can be generated by localized amino acid inhibitions along a rostrocaudal anatomical gradient within medial shell of nucleus accumbens of the rat. This can be produced by microinjections in medial shell of either the γ-aminobutyric acid (GABA)A agonist muscimol (mimicking intrinsic GABAergic inputs) or the AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) antagonist DNQX (6,7-dinitroquinoxaline-2,3-dione), disrupting corticolimbic glutamate inputs). At rostral sites in medial shell, each drug robustly stimulates appetitive eating and food intake, whereas at more caudal sites the same drugs instead produce increasingly fearful behaviors such as escape, distress vocalizations and defensive treading (an antipredator behavior rodents emit to snakes and scorpions). Previously we showed that intense motivated behaviors generated by glutamate blockade require local endogenous dopamine and can be modulated in valence by environmental ambience. Here we investigated whether GABAergic generation of intense appetitive and fearful motivations similarly depends on local dopamine signals, and whether the valence of motivations generated by GABAergic inhibition can also be retuned by changes in environmental ambience. We report that the answer to both questions is 'no'. Eating and fear generated by GABAergic inhibition of accumbens shell does not need endogenous dopamine. Also, the appetitive/fearful valence generated by GABAergic muscimol microinjections resists environmental retuning and is determined almost purely by rostrocaudal anatomical placement. These results suggest that nucleus accumbens GABAergic release of fear and eating are relatively independent of modulatory dopamine signals, and more anatomically pre-determined in valence balance than release of the same intense behaviors by glutamate disruptions.
© 2013 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures






Similar articles
-
Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear.J Neurosci. 2011 Sep 7;31(36):12866-79. doi: 10.1523/JNEUROSCI.1339-11.2011. J Neurosci. 2011. PMID: 21900565 Free PMC article.
-
Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens.J Neurosci. 2008 Jul 9;28(28):7184-92. doi: 10.1523/JNEUROSCI.4961-07.2008. J Neurosci. 2008. PMID: 18614688 Free PMC article.
-
Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption.Biol Psychiatry. 2013 Feb 15;73(4):360-70. doi: 10.1016/j.biopsych.2012.08.009. Epub 2012 Sep 13. Biol Psychiatry. 2013. PMID: 22981656 Free PMC article.
-
Desire or Dread from Nucleus Accumbens Inhibitions: Reversed by Same-Site Optogenetic Excitations.J Neurosci. 2020 Mar 25;40(13):2737-2752. doi: 10.1523/JNEUROSCI.2902-19.2020. Epub 2020 Feb 19. J Neurosci. 2020. PMID: 32075899 Free PMC article.
-
Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding.Eur J Neurosci. 2003 May;17(10):2187-200. doi: 10.1046/j.1460-9568.2003.02642.x. Eur J Neurosci. 2003. PMID: 12786986
Cited by
-
The neurobiology of alcohol consumption and alcoholism: an integrative history.Pharmacol Biochem Behav. 2013 Nov 15;113:20-37. doi: 10.1016/j.pbb.2013.10.009. Epub 2013 Oct 17. Pharmacol Biochem Behav. 2013. PMID: 24141171 Free PMC article. Review.
-
New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system.J Neurosci. 2013 Nov 6;33(45):17569-76. doi: 10.1523/JNEUROSCI.3250-13.2013. J Neurosci. 2013. PMID: 24198347 Free PMC article. Review.
-
Norepinephrine in the Medial Pre-frontal Cortex Supports Accumbens Shell Responses to a Novel Palatable Food in Food-Restricted Mice Only.Front Behav Neurosci. 2018 Jan 26;12:7. doi: 10.3389/fnbeh.2018.00007. eCollection 2018. Front Behav Neurosci. 2018. PMID: 29434542 Free PMC article.
-
Architectural Representation of Valence in the Limbic System.Neuropsychopharmacology. 2016 Jun;41(7):1697-715. doi: 10.1038/npp.2015.358. Epub 2015 Dec 9. Neuropsychopharmacology. 2016. PMID: 26647973 Free PMC article. Review.
-
Effects of Maternal Separation on Effort-based Responding for Sucrose Are Associated with c-Fos Expression in the Nucleus Accumbens Core.Neuroscience. 2024 Jan 26;537:174-188. doi: 10.1016/j.neuroscience.2023.11.030. Epub 2023 Nov 28. Neuroscience. 2024. PMID: 38036058 Free PMC article.
References
-
- Aldridge JW, Berridge KC, Herman M, Zimmer L. Neuronal coding of serial order: Syntax of grooming in the neostriatum. Psychological Science. 1993;4:391–395.
-
- Beckstead RM. Autoradiographic examination of cortico-cortical and sub-cortical projections of the mediodorsal-projection (prefrontal) cortex in the rat. Journal of Comparative Neurology. 1979;184:43–62. - PubMed
-
- Behrends JC, Lambert JDC, Jensen K. Repetitive activation of postsynaptic GABA(A) receptors by rapid, focal agonist application onto intact rat striatal neurones in vitro. Pflugers Archiv-European Journal of Physiology. 2002;443:707–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

