Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia
- PMID: 23551937
- PMCID: PMC3626545
- DOI: 10.1186/1744-8069-9-18
Electroacupuncture improves thermal and mechanical sensitivities in a rat model of postherpetic neuralgia
Abstract
Background: Electroacupuncture (EA) is effective in relieving pain in patients with postherpetic neuralgia (PHN). However, the mechanism underlying the therapeutic effect of EA in PHN is still unclear. Systemic injection of resiniferatoxin (RTX), an ultrapotent analog of TRPV1 agonist, in adult rats can reproduce the clinical symptoms of PHN by ablating TRPV1-expressing sensory neurons. In this study, we determined the beneficial effect of EA and the potential mechanisms in this rat model of PHN.
Methods: PHN was induced in rats by a single injection of RTX. Thermal hyperalgesia was tested with a radiant heat stimulus, and mechanical allodynia was quantified with von Frey filaments. TRPV1 receptors were shown by using immunofluorescence labeling. The ultrastructural changes of the sciatic nerve were assessed by electron microscopic examination. The sprouting of myelinated primary afferent terminals into the spinal dorsal horn was mapped by using the transganglionic tracer cholera toxin B-subunit (CTB).
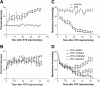
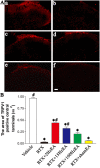
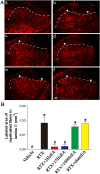
Results: RTX injection diminished thermal sensitivity and gradually induced tactile allodynia within 3 weeks. EA applied to GB30 and GB34 at 2 and 15 Hz, but not 100 Hz, significantly increased the thermal sensitivity 4 weeks after treatment and decreased the tactile allodynia 2 weeks after treatment in RTX-treated rats. EA treatment at 2 and 15 Hz recovered the loss of TRPV1-positive dorsal root ganglion neurons and their central terminals of afferent fibers in the spinal superficial dorsal horn of RTX-treated rats. Moreover, EA significantly reduced the loss of unmyelinated fibers and the damage of the myelinated nerve fibers of RTX-treated rats. Furthermore, EA at 2 and 15 Hz inhibited the sprouting of myelinated primary afferent terminals into the spinal lamina II of RTX-treated rats.
Conclusions: EA treatment improves thermal perception by recovering TRPV1-positive sensory neurons and nerve terminals damaged by RTX. EA Also reduces RTX-induced tactile allodynia by attenuating the damage of myelinated afferent nerves and their abnormal sprouting into the spinal lamina II. Our study provides new information about the mechanisms of the therapeutic actions of EA in the treatment of PHN.
Figures


Similar articles
-
Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action.J Neurosci. 2003 Apr 1;23(7):2911-9. doi: 10.1523/JNEUROSCI.23-07-02911.2003. J Neurosci. 2003. PMID: 12684478 Free PMC article.
-
Netrin-1 Contributes to Myelinated Afferent Fiber Sprouting and Neuropathic Pain.Mol Neurobiol. 2016 Oct;53(8):5640-51. doi: 10.1007/s12035-015-9482-x. Epub 2015 Oct 19. Mol Neurobiol. 2016. PMID: 26482371
-
Electroacupuncture decreases Netrin-1-induced myelinated afferent fiber sprouting and neuropathic pain through μ-opioid receptors.J Pain Res. 2019 Apr 23;12:1259-1268. doi: 10.2147/JPR.S191900. eCollection 2019. J Pain Res. 2019. PMID: 31118749 Free PMC article.
-
Vanilloid-induced conduction analgesia: selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity.Anesth Analg. 2008 Jul;107(1):271-81. doi: 10.1213/ane.0b013e318162cfa3. Anesth Analg. 2008. PMID: 18635498 Free PMC article. Review.
-
A novel approach to completely alleviate peripheral neuropathic pain in human patients: insights from preclinical data.Front Neuroanat. 2025 Jan 7;18:1523095. doi: 10.3389/fnana.2024.1523095. eCollection 2024. Front Neuroanat. 2025. PMID: 39839257 Free PMC article. Review.
Cited by
-
Acupuncture-Analgesia-Mediated Alleviation of Central Sensitization.Evid Based Complement Alternat Med. 2019 Mar 7;2019:6173412. doi: 10.1155/2019/6173412. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 30984277 Free PMC article. Review.
-
Activation and expression of μ-calpain in dorsal root contributes to RTX-induced mechanical allodynia.Mol Pain. 2017 Jan-Dec;13:1744806917719169. doi: 10.1177/1744806917719169. Mol Pain. 2017. PMID: 28714350 Free PMC article.
-
Rodent models of postherpetic neuralgia: How far have we reached?Front Immunol. 2023 Mar 20;14:1026269. doi: 10.3389/fimmu.2023.1026269. eCollection 2023. Front Immunol. 2023. PMID: 37020565 Free PMC article. Review.
-
Inhibition of GABAergic Neurons and Excitation of Glutamatergic Neurons in the Ventrolateral Periaqueductal Gray Participate in Electroacupuncture Analgesia Mediated by Cannabinoid Receptor.Front Neurosci. 2019 May 17;13:484. doi: 10.3389/fnins.2019.00484. eCollection 2019. Front Neurosci. 2019. PMID: 31156369 Free PMC article.
-
Neuregulin1-ErbB4 Signaling in Spinal Cord Participates in Electroacupuncture Analgesia in Inflammatory Pain.Front Neurosci. 2021 Jan 28;15:636348. doi: 10.3389/fnins.2021.636348. eCollection 2021. Front Neurosci. 2021. PMID: 33584196 Free PMC article.
References
-
- Baron R, Saguer M. Postherpetic neuralgia. Are C-nociceptors involved in signalling and maintenance of tactile allodynia? Brain. 1993;116(Pt 6):1477–1496. - PubMed
-
- Grachev Iu V, Kukushkin ML, Sudarikov AP, Zhuravlev VF, Gerasimenko M. Clinical course and treatment of herpetic trigeminal ganglionic neuropathy. Zh Nevrol Psikhiatr Im S S Korsakova. 1998;98:4–8. - PubMed
-
- Wang CY, Fang JQ. Analysis on therapeutic effect of variable-frequency electroacupuncture combined with herbal-moxa moxibustion for post-zoster neuralgia. Zhen Ci Yan Jiu. 2012;37:64–66. - PubMed
-
- Rowbotham MC, Fields HL. The relationship of pain, allodynia and thermal sensation in post-herpetic neuralgia. Brain. 1996;119(Pt 2):347–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

