Removal of half the sympathetic innervation does not reduce vasoconstrictor responses in rat tail artery
- PMID: 23551946
- PMCID: PMC3690691
- DOI: 10.1113/jphysiol.2012.250365
Removal of half the sympathetic innervation does not reduce vasoconstrictor responses in rat tail artery
Abstract
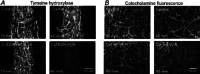
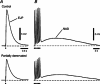
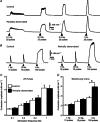
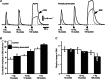
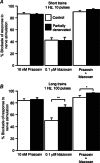
Following reinnervation of denervated rat tail arteries, nerve-evoked contractions are at least as large as those evoked in normally innervated arteries despite a much lower nerve terminal density. Here nerve-evoked contractions have been investigated after transection of half the sympathetic innervation of normal tail arteries. After 1 week, the noradrenergic plexus 50-70 mm along the tail was about half as dense as control. Excitatory junction potentials recorded in smooth muscle cells of arterial segments isolated in vitro were half their normal amplitude. Surprisingly, nerve-evoked contractions of isometrically mounted segments were not reduced in amplitude, as was also the case after only 3 days. After 1 week, enhancement of nerve-evoked contractions by blocking either neuronal re-uptake of noradrenaline with desmethylimipramine or prejunctional α2-adrenoceptors with idazoxan was similar to control, suggesting that these mechanisms are matched to the number of innervating axons. The relative contribution of postjunctional α2-adrenoceptors to contractions evoked by long trains of stimuli was enhanced but that of α1-adrenoceptors was unchanged. Transiently, sensitivity to the α1-adrenoceptor agonist phenylephrine was slightly increased. After 7 weeks, amplitudes of nerve-evoked contractions remained similar to control, and sensitivity to phenylephrine had recovered but that to the α2-adrenoceptor agonist clonidine was slightly raised. The normal amplitude of nerve-evoked contractions after partial denervation is only partly explained by the greater contribution of α2-adrenoceptors. While the post-receptor mechanisms activated by nerve-released transmitter may be modified to amplify the contractions after partial denervation, our findings suggest that these mechanisms are normally saturated, at least in this artery.
Figures


Similar articles
-
Slow and incomplete sympathetic reinnervation of rat tail artery restores the amplitude of nerve-evoked contractions provided a perivascular plexus is present.Am J Physiol Heart Circ Physiol. 2011 Feb;300(2):H541-54. doi: 10.1152/ajpheart.00834.2010. Epub 2010 Nov 19. Am J Physiol Heart Circ Physiol. 2011. PMID: 21097663
-
Sympathetic vasoconstriction is potentiated in arteries caudal but not rostral to a spinal cord transection in rats.J Neurotrauma. 2010 Nov;27(11):2077-89. doi: 10.1089/neu.2010.1468. J Neurotrauma. 2010. PMID: 20822463
-
Tail arteries from chronically spinalized rats have potentiated responses to nerve stimulation in vitro.J Physiol. 2004 Apr 15;556(Pt 2):545-55. doi: 10.1113/jphysiol.2003.056424. Epub 2004 Feb 6. J Physiol. 2004. PMID: 14766944 Free PMC article.
-
Sympathetic neuromuscular transmission in rat tail artery: a study based on electrochemical, electrophysiological and mechanical recording.Acta Physiol Scand Suppl. 1993;610:1-58. Acta Physiol Scand Suppl. 1993. PMID: 8397469 Review.
-
Sympathetic Nervous System and Atherosclerosis.Int J Mol Sci. 2023 Aug 23;24(17):13132. doi: 10.3390/ijms241713132. Int J Mol Sci. 2023. PMID: 37685939 Free PMC article. Review.
Cited by
-
Endothelial and Neuronal Nitric Oxide Activate Distinct Pathways on Sympathetic Neurotransmission in Rat Tail and Mesenteric Arteries.PLoS One. 2015 Jun 15;10(6):e0129224. doi: 10.1371/journal.pone.0129224. eCollection 2015. PLoS One. 2015. PMID: 26075386 Free PMC article.
-
Increased peripherin in sympathetic axons innervating plantar metatarsal arteries in STZ-induced type I diabetic rats.Front Neurosci. 2014 May 7;8:99. doi: 10.3389/fnins.2014.00099. eCollection 2014. Front Neurosci. 2014. PMID: 24847201 Free PMC article.
References
-
- Al Dera H, Habgood MD, Furness JB, Brock JA. Prominent contribution of L-type Ca2+ channels to cutaneous neurovascular transmission that is revealed after spinal cord injury augments vasoconstriction. Am J Physiol Heart Circ Physiol. 2012;302:H752–762. - PubMed
-
- Andres KH, von During M, Janig W, Schmidt RF. Degeneration patterns of postganglionic fibers following sympathectomy. Anat Embryol (Berl) 1985;172:133–143. - PubMed
-
- Bao JX, Gonon F, Stjärne L. Frequency- and train length-dependent variation in the roles of postjunctional α1- and α2-adrenoceptors for the field stimulation-induced neurogenic contraction of rat tail artery. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:601–616. - PubMed
-
- Bradley E, Law A, Bell D, Johnson CD. Effects of varying impulse number on cotransmitter contributions to sympathetic vasoconstriction in rat tail artery. Am J Physiol Heart Circ Physiol. 2003;284:H2007–2014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

