Pinellia pedatisecta agglutinin interacts with the methylosome and induces cancer cell death
- PMID: 23552401
- PMCID: PMC3503292
- DOI: 10.1038/oncsis.2012.30
Pinellia pedatisecta agglutinin interacts with the methylosome and induces cancer cell death
Abstract
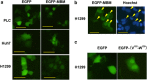
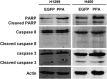
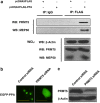
Pinellia pedatisecta agglutinin (PPA) is a specific mannose-binding plant lectin accumulated in the tuber of P. pedatisecta. In the work presented, the cytotoxicity of PPA to cancer cells was investigated through exogenous expression. A PPA gene was transduced into normal and cancer cell lines through plasmid vectors, and the effect of PPA expression was examined. Results showed that PPA translocated into the nucleus, colocalized with DNA and induced cell death. A mannose-binding motif and a V(103)-W(130) region directed the nuclear translocation of PPA. Coprecipitation, mass spectrometry and western blotting analysis further indentified that PPA was associated with the methylosome, which contains methylosome protein 50 and protein arginine methyltransferase 5 (PRMT5). Knockdown of PRMT5 significantly inhibited the PPA-induced cell death, suggesting that PPA used the methylosome as a target. Furthermore, Ad.surp-PPA, an adenovirus vector in which the PPA gene was controlled by a survivin promoter (surp), selectively inhibited the proliferation of cancer cell lines. Taken together, the expression of PPA gene elicited significant cytotoxicity to cancer cells through targeting the methylosome and might be developed into a novel agent in cancer gene therapy.
Figures



Similar articles
-
cDNA cloning and expression analysis of a mannose-binding lectin from Pinellia pedatisecta.J Biosci. 2007 Mar;32(2):241-9. doi: 10.1007/s12038-007-0024-1. J Biosci. 2007. PMID: 17435316
-
Genomic cloning and characterization of a PPA gene encoding a mannose-binding lectin from Pinellia pedatisecta.Biocell. 2006 Apr;30(1):15-25. Biocell. 2006. PMID: 16845824
-
Expression of Pinellia pedatisecta Lectin Gene in Transgenic Wheat Enhances Resistance to Wheat Aphids.Molecules. 2018 Mar 24;23(4):748. doi: 10.3390/molecules23040748. Molecules. 2018. PMID: 29587341 Free PMC article.
-
Mannose-exposing myeloid leukemia cells detected by the sCAR-PPA fusion protein.Int J Hematol. 2009 Jun;89(5):611-7. doi: 10.1007/s12185-009-0308-3. Epub 2009 Apr 18. Int J Hematol. 2009. PMID: 19377843
-
Pinellia pedatisecta agglutinin-based lectin blot analysis distinguishes between glycosylation patterns in various cancer cell lines.Oncol Lett. 2014 Aug;8(2):837-840. doi: 10.3892/ol.2014.2201. Epub 2014 May 30. Oncol Lett. 2014. PMID: 25013506 Free PMC article.
Cited by
-
Cellular localization of protein arginine methyltransferase-5 correlates with grade of lung tumors.Diagn Pathol. 2013 Dec 10;8:201. doi: 10.1186/1746-1596-8-201. Diagn Pathol. 2013. PMID: 24326178 Free PMC article.
-
Plant protein-based hydrophobic fine and ultrafine carrier particles in drug delivery systems.Crit Rev Biotechnol. 2018 Feb;38(1):47-67. doi: 10.1080/07388551.2017.1312267. Epub 2017 Apr 24. Crit Rev Biotechnol. 2018. PMID: 28434263 Free PMC article. Review.
-
Rhizoma Pinelliae trypsin inhibitor separation, purification and inhibitory activity on the proliferation of BGC-823 gastric adenocarcinoma cells.Exp Ther Med. 2014 Jul;8(1):248-254. doi: 10.3892/etm.2014.1701. Epub 2014 May 8. Exp Ther Med. 2014. PMID: 24944630 Free PMC article.
-
CD123 targeting oncolytic adenoviruses suppress acute myeloid leukemia cell proliferation in vitro and in vivo.Blood Cancer J. 2014 Mar 21;4(3):e194. doi: 10.1038/bcj.2014.15. Blood Cancer J. 2014. PMID: 24658372 Free PMC article.
-
The high-quality Pinellia pedatisecta genome reveals a key role of tandem duplication in the expansion of its agglutinin genes.Hortic Res. 2022 Dec 30;10(3):uhac289. doi: 10.1093/hr/uhac289. eCollection 2023 Mar. Hortic Res. 2022. PMID: 36938569 Free PMC article. No abstract available.
References
-
- Sharon N, Lis H. Lectins as cell recognition molecules. Science. 1989;246:227–234. - PubMed
-
- Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiol. 2004;14:53R–62R. - PubMed
-
- Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. J Biol Chem. 2007;282:2753–2764. - PubMed
LinkOut - more resources
Full Text Sources

