Toscana virus NSs protein inhibits the induction of type I interferon by interacting with RIG-I
- PMID: 23552410
- PMCID: PMC3676095
- DOI: 10.1128/JVI.03129-12
Toscana virus NSs protein inhibits the induction of type I interferon by interacting with RIG-I
Abstract
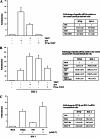
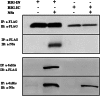
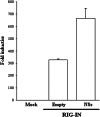
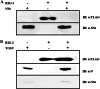
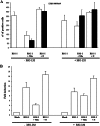
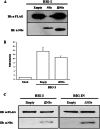
Toscana virus (TOSV) is a phlebovirus, of the Bunyaviridae family, that is responsible for central nervous system (CNS) injury in humans. Previous data have shown that the TOSV NSs protein is a gamma interferon (IFN-β) antagonist when transiently overexpressed in mammalian cells, inhibiting IRF-3 induction (G. Gori Savellini, F. Weber, C. Terrosi, M. Habjan, B. Martorelli, and M. G. Cusi, J. Gen. Virol. 92:71-79, 2011). In this study, we investigated whether an upstream sensor, which has a role in the signaling cascade leading to the production of type I IFN, was involved. We found a significant decrease in RIG-I protein levels in cells overexpressing TOSV NSs, suggesting that the nonstructural protein interacts with RIG-I and targets it for proteasomal degradation. In fact, the MG-132 proteasome inhibitor was able to restore IFN-β promoter activation in cells expressing NSs, demonstrating the existence of an evasion mechanism based on inhibition of the RIG-I sensor. Furthermore, a C-terminal truncated NSs protein (ΔNSs), although able to interact with RIG-I, did not affect the RIG-I-mediated IFN-β promoter activation, suggesting that the NSs domains responsible for RIG-I-mediated signaling and interaction with RIG-I are mapped on different regions. These results contribute to identify a novel mechanism for bunyaviruses by which TOSV NSs counteracts the early IFN response.
Figures
Similar articles
-
Ubiquitin and Not Only Unfolded Domains Drives Toscana Virus Non-Structural NSs Protein Degradation.Viruses. 2020 Oct 12;12(10):1153. doi: 10.3390/v12101153. Viruses. 2020. PMID: 33053780 Free PMC article.
-
Truncation of the C-terminal region of Toscana Virus NSs protein is critical for interferon-β antagonism and protein stability.Virology. 2015 Dec;486:255-62. doi: 10.1016/j.virol.2015.09.021. Epub 2015 Oct 27. Virology. 2015. PMID: 26474372
-
Toscana virus non-structural protein NSs acts as E3 ubiquitin ligase promoting RIG-I degradation.PLoS Pathog. 2019 Dec 9;15(12):e1008186. doi: 10.1371/journal.ppat.1008186. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31815967 Free PMC article.
-
Toscana virus NSs protein promotes degradation of double-stranded RNA-dependent protein kinase.J Virol. 2013 Apr;87(7):3710-8. doi: 10.1128/JVI.02506-12. Epub 2013 Jan 16. J Virol. 2013. PMID: 23325696 Free PMC article.
-
Phleboviruses and the Type I Interferon Response.Viruses. 2016 Jun 22;8(6):174. doi: 10.3390/v8060174. Viruses. 2016. PMID: 27338447 Free PMC article. Review.
Cited by
-
Ubiquitin and Not Only Unfolded Domains Drives Toscana Virus Non-Structural NSs Protein Degradation.Viruses. 2020 Oct 12;12(10):1153. doi: 10.3390/v12101153. Viruses. 2020. PMID: 33053780 Free PMC article.
-
Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors.Cell Mol Immunol. 2021 Mar;18(3):539-555. doi: 10.1038/s41423-020-00602-7. Epub 2021 Jan 18. Cell Mol Immunol. 2021. PMID: 33462384 Free PMC article. Review.
-
Mapping of Transcription Termination within the S Segment of SFTS Phlebovirus Facilitated Generation of NSs Deletant Viruses.J Virol. 2017 Jul 27;91(16):e00743-17. doi: 10.1128/JVI.00743-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28592543 Free PMC article.
-
Viruses utilize ubiquitination systems to escape TLR/RLR-mediated innate immunity.Front Immunol. 2022 Nov 25;13:1065211. doi: 10.3389/fimmu.2022.1065211. eCollection 2022. Front Immunol. 2022. PMID: 36505476 Free PMC article. Review.
-
Attenuation of pathogenic Rift Valley fever virus strain through the chimeric S-segment encoding sandfly fever phlebovirus NSs or a dominant-negative PKR.Virulence. 2016 Nov 16;7(8):871-881. doi: 10.1080/21505594.2016.1195528. Epub 2016 Jun 1. Virulence. 2016. PMID: 27248570 Free PMC article.
References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

