Vesicular stomatitis virus variants selectively infect and kill human melanomas but not normal melanocytes
- PMID: 23552414
- PMCID: PMC3676084
- DOI: 10.1128/JVI.03311-12
Vesicular stomatitis virus variants selectively infect and kill human melanomas but not normal melanocytes
Abstract
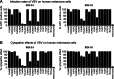
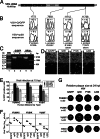
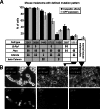
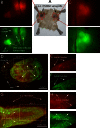
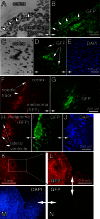
Metastatic malignant melanoma remains one of the most therapeutically challenging forms of cancer. Here we test replication-competent vesicular stomatitis viruses (VSV) on 19 primary human melanoma samples and compare these infections with those of normal human melanocyte control cells. Even at a low viral concentration, we found a strong susceptibility to viral oncolysis in over 70% of melanomas. In contrast, melanocytes displayed strong resistance to virus infection and showed complete protection by interferon. Several recombinant VSVs were compared, and all infected and killed most melanomas with differences in the time course with increasing rates of melanoma infection, as follows: VSV-CT9-M51 < VSV-M51 < VSV-G/GFP < VSV-rp30. VSV-rp30 sequencing revealed 2 nonsynonymous mutations at codon positions P126 and L223, both of which appear to be required for the enhanced phenotype. VSV-rp30 showed effective targeting and infection of multiple subcutaneous and intracranial melanoma xenografts in SCID mice after tail vein virus application. Sequence analysis of mutations in the melanomas used revealed that BRAF but not NRAS gene mutation status was predictive for enhanced susceptibility to infection. In mouse melanoma models with specific induced gene mutations including mutations of the Braf, Pten, and Cdkn2a genes, viral infection correlated with the extent of malignant transformation. Similar to human melanocytes, mouse melanocytes resisted VSV-rp30 infection. This study confirms the general susceptibility of the majority of human melanoma types for VSV-mediated oncolysis.
Figures


Similar articles
-
Variation in susceptibility of human malignant melanomas to oncolytic vesicular stomatitis virus.Surgery. 2013 Mar;153(3):333-43. doi: 10.1016/j.surg.2012.09.003. Epub 2012 Oct 25. Surgery. 2013. PMID: 23102637 Free PMC article.
-
Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells.J Virol. 2010 Feb;84(3):1563-73. doi: 10.1128/JVI.02040-09. Epub 2009 Nov 11. J Virol. 2010. PMID: 19906910 Free PMC article.
-
Experimental Evolution Generates Novel Oncolytic Vesicular Stomatitis Viruses with Improved Replication in Virus-Resistant Pancreatic Cancer Cells.J Virol. 2020 Jan 17;94(3):e01643-19. doi: 10.1128/JVI.01643-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694943 Free PMC article.
-
Oncolytic Therapy of Solid Tumors by Modified Vesicular Stomatitis Virus.DNA Cell Biol. 2024 Feb;43(2):57-60. doi: 10.1089/dna.2023.0368. Epub 2023 Dec 11. DNA Cell Biol. 2024. PMID: 38079267 Review.
-
Oncotargeting by Vesicular Stomatitis Virus (VSV): Advances in Cancer Therapy.Viruses. 2018 Feb 23;10(2):90. doi: 10.3390/v10020090. Viruses. 2018. PMID: 29473868 Free PMC article. Review.
Cited by
-
Chemotherapy and Oncolytic Virotherapy: Advanced Tactics in the War against Cancer.Front Oncol. 2014 Jun 11;4:145. doi: 10.3389/fonc.2014.00145. eCollection 2014. Front Oncol. 2014. PMID: 24967214 Free PMC article. Review.
-
Employing the Oncolytic Vesicular Stomatitis Virus in Cancer Virotherapy: Resistance and Clinical Considerations.Viruses. 2024 Dec 25;17(1):16. doi: 10.3390/v17010016. Viruses. 2024. PMID: 39861805 Free PMC article. Review.
-
Mucin-Like Domain of Ebola Virus Glycoprotein Enhances Selective Oncolytic Actions against Brain Tumors.J Virol. 2020 Mar 31;94(8):e01967-19. doi: 10.1128/JVI.01967-19. Print 2020 Mar 31. J Virol. 2020. PMID: 32051271 Free PMC article.
-
Experimental virus evolution in cancer cell monolayers, spheroids, and tissue explants.Virus Evol. 2021 May 6;7(1):veab045. doi: 10.1093/ve/veab045. eCollection 2021 Jan. Virus Evol. 2021. PMID: 34040797 Free PMC article.
-
Experimental evolution of an oncolytic vesicular stomatitis virus with increased selectivity for p53-deficient cells.PLoS One. 2014 Jul 10;9(7):e102365. doi: 10.1371/journal.pone.0102365. eCollection 2014. PLoS One. 2014. PMID: 25010337 Free PMC article.
References
-
- Mocellin S, Pasquali S, Rossi CR, Nitti D. 2010. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J. Natl. Cancer Inst. 102:493–501 - PubMed
-
- Carlino MS, Fogarty GB, Long GV. 2012. Treatment of melanoma brain metastases: a new paradigm. Cancer J. 18:208–212 - PubMed
-
- Sloan AE, Nock CJ, Einstein DB. 2009. Diagnosis and treatment of melanoma brain metastasis: a literature review. Cancer Control 16:248–255 - PubMed
-
- Trinh VA, Hwu WJ. 2012. Ipilimumab in the treatment of melanoma. Expert Opin. Biol. Ther. 12:773–782 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

